Hirudo polypeptide with functions of antithrombus and brain nerve cell protection and application of hirudo polypeptide
A brain nerve cell, leech polypeptide technology, applied in extracellular fluid diseases, peptides, blood diseases, etc., can solve problems such as unclear active ingredients, achieve the effect of improving neurobehavior, reducing the formation of thrombus in the body, and being easy to prepare in large quantities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1 Separation of polypeptide whitmantides A-C
[0056] (1) Leech dried 3 kg of the whole body, washed with physiological saline, soaked and crushed the homogenate, the homogenate was frozen and thawed to obtain the extract. The obtained extract is processed by an ultrafiltration membrane with a molecular weight cut-off of 50KDa, and the ultrafiltrate with a molecular weight of less than 50KDa is collected. After the obtained ultrafiltrate is solid-phase desalinated, it is concentrated under reduced pressure or freeze-dried to obtain the total extract (13g) . At 4~8℃, the total extract was separated by gel Sephadex G25. Ultra-high performance liquid chromatography-mass spectrometry (UPLC-MS) method was used for analysis and tracking detection, and the fractions containing peptide compounds were collected, concentrated under reduced pressure or vacuum dried to obtain peptide-enriched fractions (10g).
[0057] (2) Take the polypeptide-enriched part in step (1) and perf...
Embodiment 2
[0061] Example 2 Structural characterization of the polypeptide whitmantide A
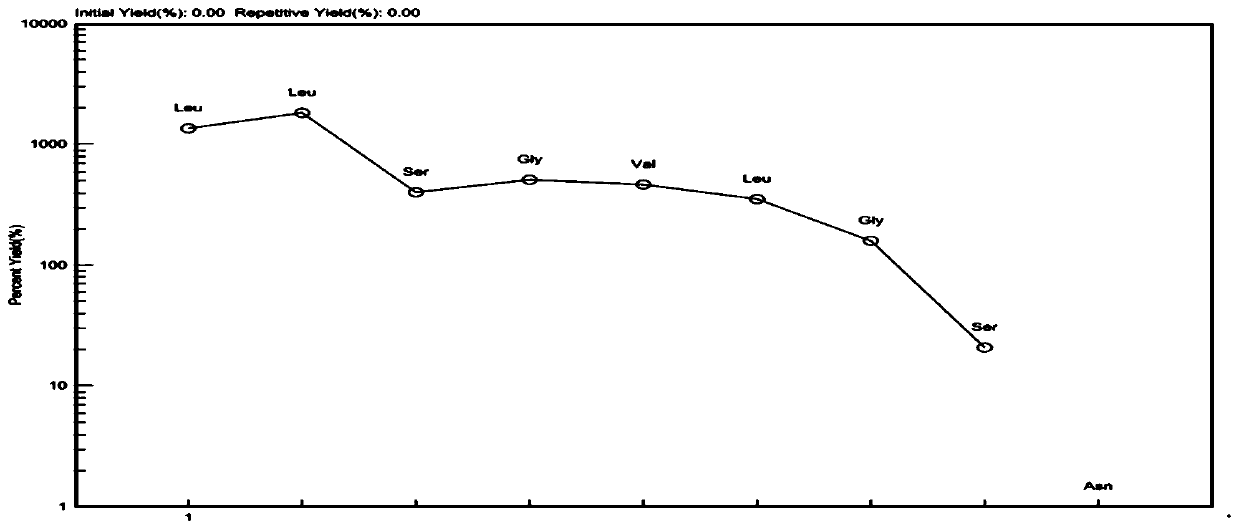
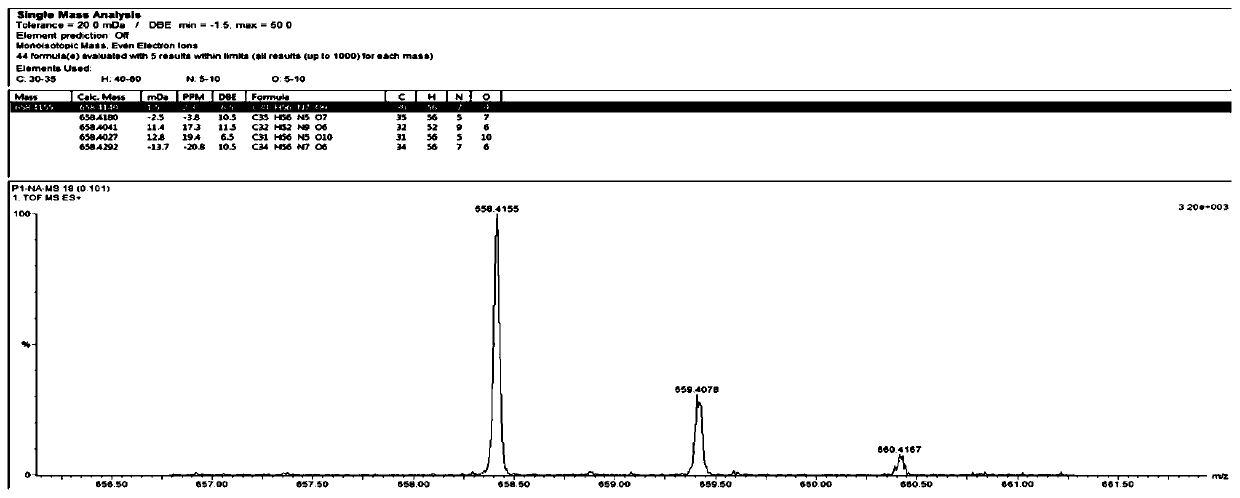
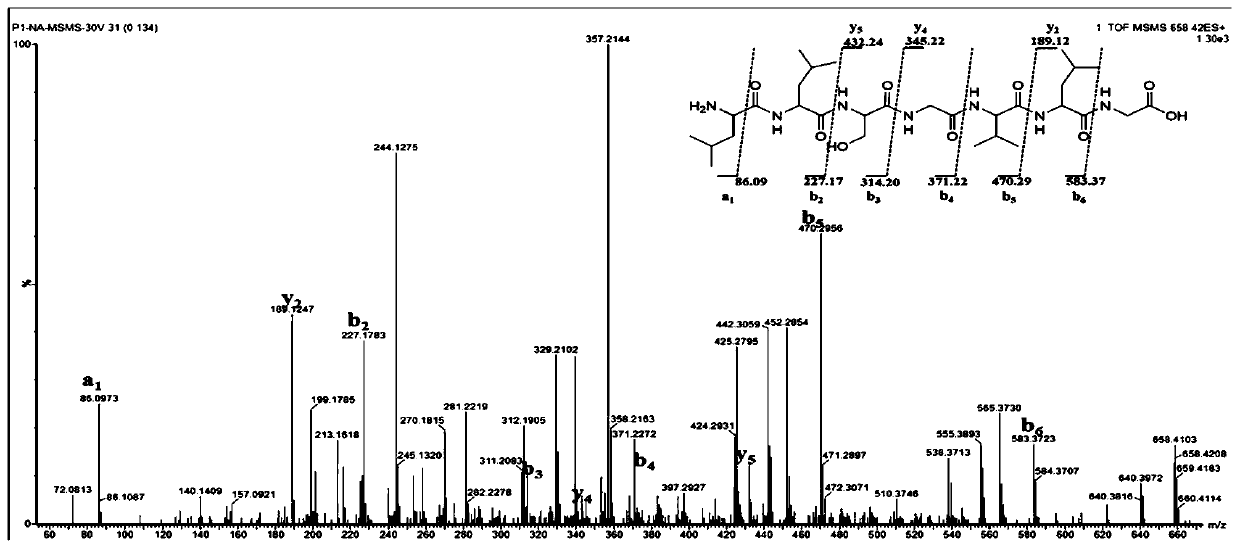
[0062] The polypeptide whitmantide A prepared in Example 1 is an amorphous powder with UV (H 2 O)λ max (logε): 195(3.20). IR(KBr)ν max :3275.5,2960.2,1657.52,1541.81cm -1 . HR-ESI-MS m / z 658.4155[M+H] + (calcd forC 30 H 56 N 7 O 9 :658.4140). The Edman degradation method was used to sequence the N-terminal of whitmantide A, and the amino acid sequence was determined to be NH 2 -Leu-Leu-Ser-Gly-Val-Leu-Gly-COOH. The MS / MS secondary mass spectrum showed that the a, b, and y ion fragments of the compound were consistent with its amino acid sequence. The above results further verified the amino acid sequence of whitmantide A. Marfey method analysis showed that whitmantide A contained two D-type leucine and one L-type leucine, and other chiral amino acids were all L-type. The absolute configuration of whitmantide A was determined by solid-phase synthesis. There are three arrangements for the positions...
Embodiment 3
[0063] Example 3 Structural characterization of the polypeptide whitmantide B
[0064] The polypeptide whitmantide B prepared in Example 1 is an amorphous powder, UV (H 2 O)λ max (logε): 196(3.18). IR(KBr)ν max :3275.5,2957.3,1640.16,1544.7,1132.97cm -1 ; HR-ESI-MS m / z715.4331[M+H] + (calcd for C 32 H 59 N 8 O 10 :715.4354). The Edman degradation method was used to sequence the N-terminal of whitmantide B, and the amino acid sequence was determined to be NH 2 -Leu-Leu-Ser-Gly-Val-Leu-Gly-Gly-COOH. The MS / MS secondary mass spectrum showed that the a, b, and y ion fragments of the compound were consistent with its amino acid sequence. The above results further verified the amino acid sequence of whitmantide B. The Marfey method analysis results show that whitmantide B contains two D-type leucine and one L-type leucine, and the other chiral amino acids are all L-type. The absolute configuration of whitmantide B was determined by solid-phase synthesis. There are three arrangement...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Average granularity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com