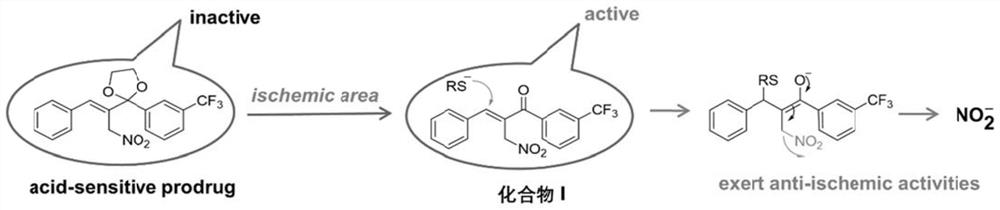
Organic nitrite donor ketal type prodrug as well as preparation method and medical application thereof
A pharmacy and drug technology, applied in the application of myocardial ischemia and pulmonary arterial hypertension drugs, the preparation of prevention or treatment of cerebral ischemia, the preparation of organic nitrite donor ketal-type prodrugs, can solve the problem of damage, unproductive Ischemic protection, protein nitration, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1: Preparation of (E)-2-nitromethyl-3-phenyl-1-(3-(trifluoromethyl)phenyl)prop-2-ene-1-ketal (Compound I)
[0034]
[0035] (a) put (NH 4 ) 2 S 2 O8 , p-amyl alcohol and dibenzylamine were placed in Schlenk flasks, 3-trifluoromethyl Propiophenone was added respectively, N 2 Replace, and react at 120°C for 24-30h; the reaction solution gradually changes from white to yellow. After the reaction was completed, water was added to quench, then extracted with ethyl acetate (100 mL), the organic layer was washed three times with water and saturated brine each, dried over anhydrous sodium sulfate, concentrated, and subjected to column chromatography (petroleum ether / ethyl acetate=100 / 1, v / v) separation and purification to obtain compound IV. Product IV was a colorless oil in 75% yield. 1 H NMR (300MHz, CDCl 3 )δ8.00(s, 1H), 7.92(d, J=7.7Hz, 1H), 7.81(d, J=7.6Hz, 1H), 7.60(t, J=7.7Hz, 1H), 7.43(d, J=4.2Hz, 4H), 7.40-7.34(m, 1H), 7.16(s, 1H), 2.29(s, 3H). 13 C...
Embodiment 2
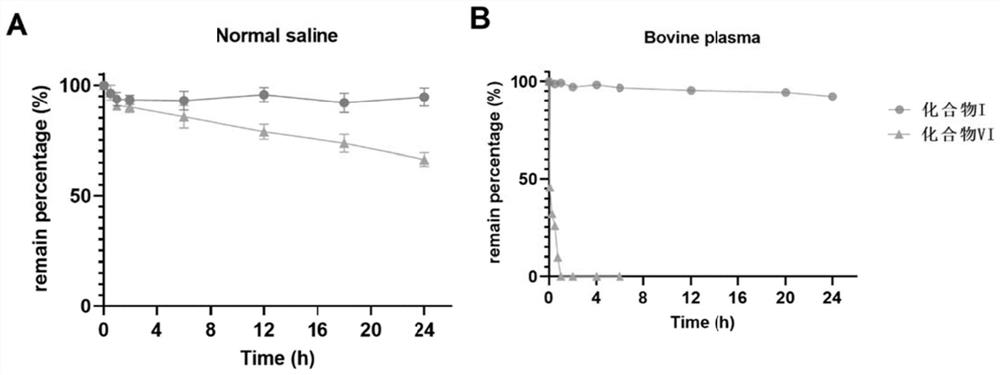
[0039] Example 2: Plasma stability test of ketal-type prodrug (Compound I)
[0040] 1. Test method
[0041] A certain concentration (200 μM) of compound I and compound VI were dissolved in bovine plasma (5% DMSO assisted solubilization), incubated at 37.4 °C, and samples were taken at regular intervals, and the peak areas of the two compounds were recorded by HPLC, so as to carry out the analysis. For comparison, Compound VI is a positive control.
[0042] 2. Test results
[0043] The result is as figure 2 shown. The results showed that Compound VI was rapidly degraded in plasma, and the degradation was completed within 2 hours; the ketal prodrug I significantly improved the plasma stability of the original drug. Compound I has good stability, and only 7.8% degraded within 24h; These results suggest that the ketal-type prodrug has better plasma stability and is significantly stronger than compound VI.
Embodiment 3
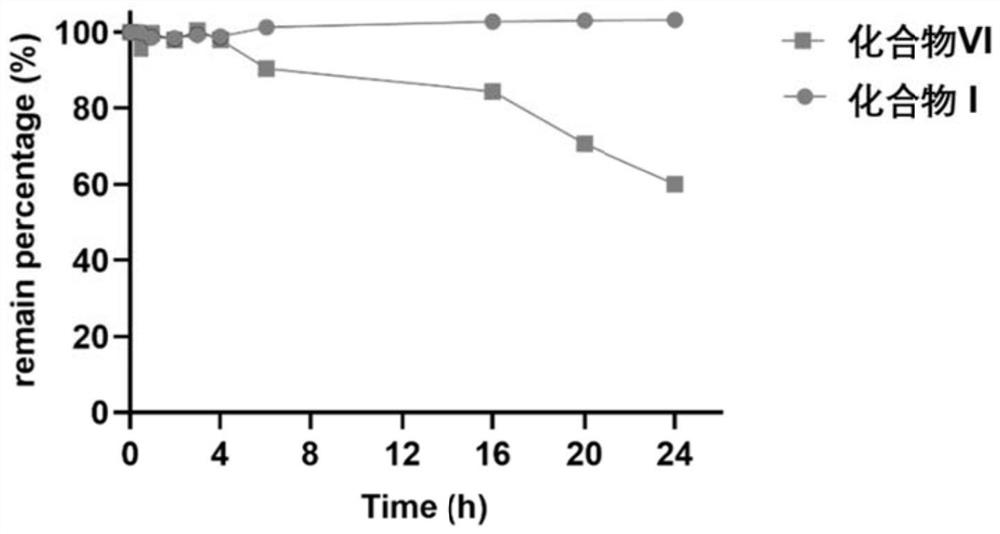
[0044] Example 3: Stability of a ketal-type prodrug (Compound I) in the presence of a nucleophile.
[0045] 1. Test method
[0046] Equal concentrations of N-acetylcysteine (200 μM) were co-incubated with Compound VI and Compound I at 37.4° C., and the concentration of each compound was determined at each time point using the same method described above.
[0047] 2. Test results
[0048] like image 3 As shown, compound VI can be gradually degraded with time, while compound I has better stability and hardly degrades in the presence of nucleophile. The above results prove that the protection of the carbonyl group in the α,β-unsaturated ketone structure of compound I can effectively reduce its ability to be attacked by nucleophiles and improve the stability.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com