Method for detecting trace genotoxic impurities in voriconazole
A technology of genotoxicity and voriconazole, which is applied in the detection field of genotoxic impurities, can solve the problems of inability to solve low-level quantitative detection and no obvious improvement in ultraviolet absorption intensity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
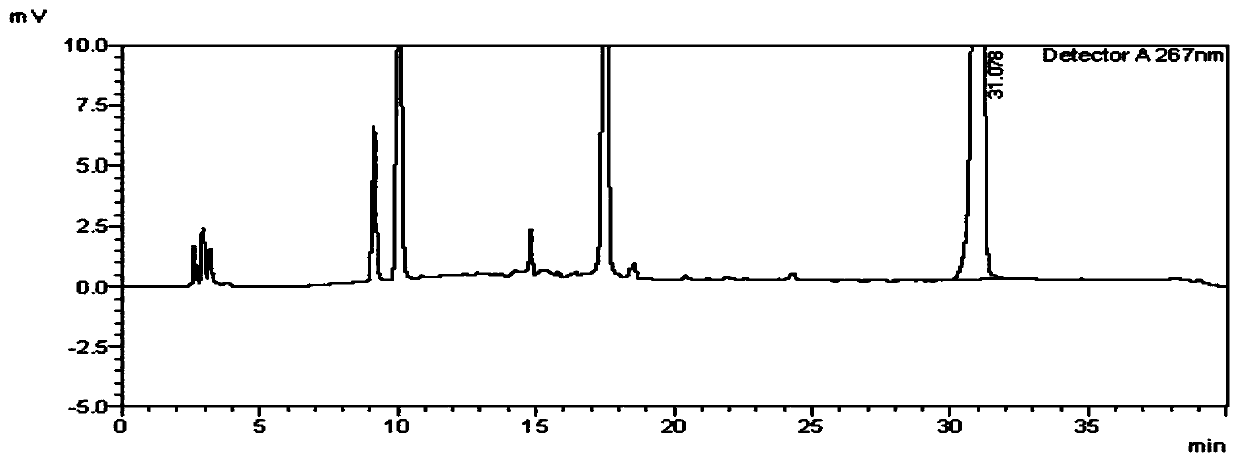
[0074] Example 1: Detection of genotoxic impurity IN-001 in voriconazole bulk drug
[0075] (1) Instruments and chromatographic conditions used:
[0076] Instrument: high performance liquid chromatography, Shimadzu, LC20AT
[0077] Column: Agilent C 18 , particle size 5μm, inner diameter 4.6*250mm
[0078] Detection wavelength: 267nm
[0079] Injection volume: 20μL
[0080] Column temperature: 40°C
[0081] Mobile phase A: 0.1% formic acid solution; Mobile phase B: acetonitrile
[0082] Flow rate: 1mL / min
[0083] The elution gradient is shown in Table 1:
[0084] Table 1
[0085] time (min) Mobile phase A(%) Mobile phase B(%) 0 80 20 10~25 20 80 25~33 10 90 33~35 10 90 35~40 80 20 40 80 20
[0086] (2) Solution preparation
[0087] Formic acid solution: Take 1mL of formic acid into 1000mL of water and mix well.
[0088] Preparation of biphenyl-4-thiophenol solution: Accurately weigh 10mg of biphenyl-4-thiophenol i...
Embodiment 2- Embodiment 9
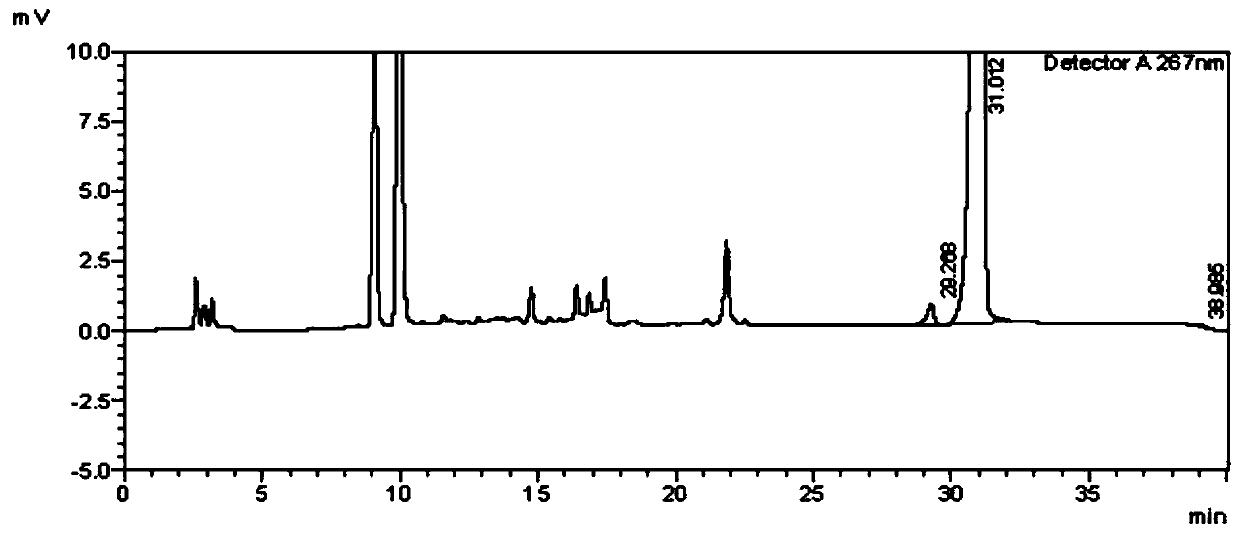
[0122] Detection of genotoxic impurity IN-001 in voriconazole API under different detection conditions
[0123] In Example 2-Example 9, except for the following conditions, other parameters and operations are the same as in Example 1, and the specific test conditions and test results are shown in Table 4.
[0124] Table 4
[0125]
[0126] Comparing the above experimental results with the results of Example 1, it can be seen that the detection method of the present invention has good stability and high detection accuracy, and can be used for quality control of voriconazole bulk drug.
Embodiment 10~ Embodiment 14
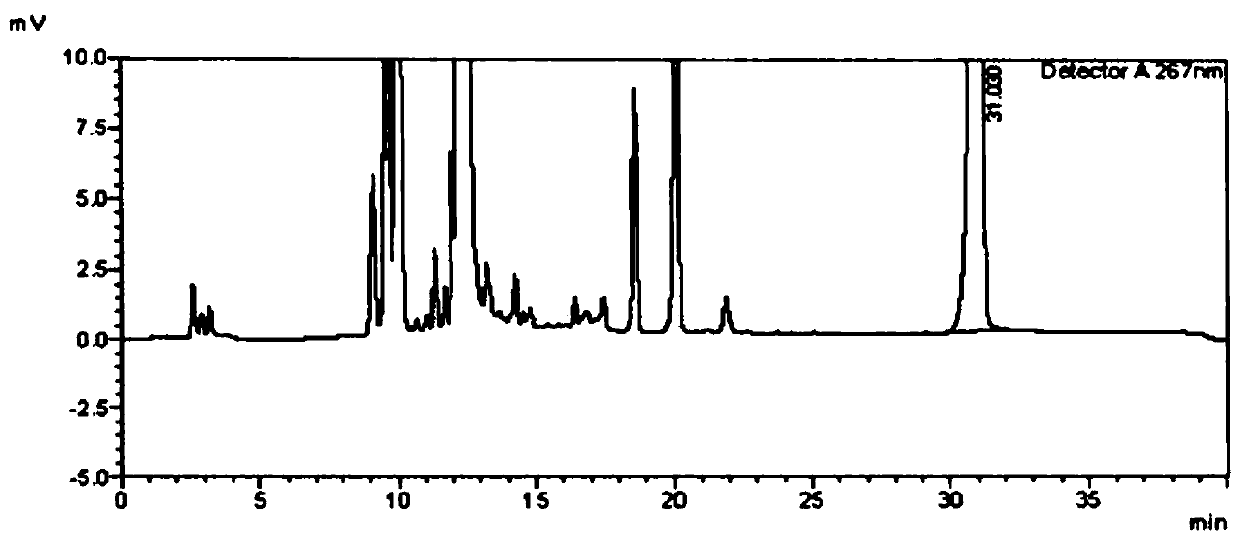
[0128] Detection of genotoxic impurity IN-001 in voriconazole API with different additions of derivatization reagents
[0129] The applicant also conducted research on impurity detection methods under the conditions of different additions of derivatization reagents. With reference to the operation method of Example 1, the amount of biphenyl-4-thiophenol solution taken in the blank solution, contrast solution, need testing solution and spiked need testing solution is respectively 150 μ L, 200 μ L, 300 μ L, 500 μ L and 1 mL, and other Parameters and operation steps are all the same as in Example 1, and are tested.
[0130] Comparing the experimental results with the results of Example 1, it is found that when the amount of derivatization reagent added is large, such as 500 μL and 1 mL, the adjacent peaks interfere with the derivatization product peak, although the derivatization product peak can be detected, but its quantitative Accuracy decreased; when the amount of derivatiza...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com