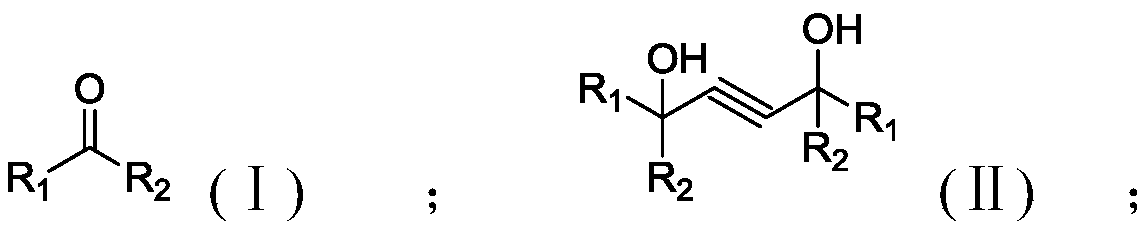
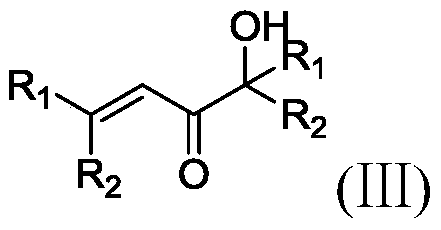
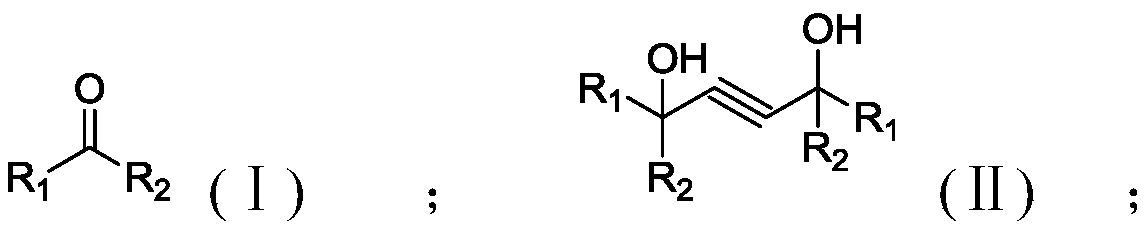
Method for preparation of alpha-hydroxy ketone from ethynylation reaction byproduct
A by-product, ethynylation technology, applied in the preparation of carbon-based compounds, organic compounds, chemical instruments and methods, etc., can solve the problems of catalyst life and catalytic selectivity, complex residual liquid components, and difficult product purification. Achieve the effects of stable product composition, high selectivity, mild and convenient conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Embodiment 1 raw material pretreatment 1
[0061] Get 6-methyl-5-hepten-2-ketone ynylation back tower still liquid 1000g and carry out vacuum distillation treatment, tower still liquid is composed of: w (dehydrolinalool)=22.8%, w (6-methanol) Base-5-hepten-2-one acetylenic diol) = 57.9%, the others are mainly polymer impurities. The decompression distillation vacuum degree is 200Pa (absolute pressure), 180 ℃ of tower still temperature, and tower top obtains the dehydrolinalool 226g that purity is 98.4% altogether. The vacuum degree is adjusted to 100Pa, the temperature of the tower kettle is 260° C., and the purity of the tower top is 575 g of 6-methyl-5-heptene-2-one acetylenic diol with a purity of 98.2%, wherein the content of dehydrolinalool is 0.94%. , The polymer impurity content is lower than 1ppm (not detected). The material obtained at the top of the tower is a by-product after pretreatment.
Embodiment 2
[0062] Embodiment 2 raw material pretreatment 2
[0063] Get 1000g of the tower still liquid after geranyl acetone is acetylated and carry out vacuum distillation treatment, the tower still liquid is composed of: w (Nerolidol)=15.6%, w (geranyl acetonyne diol)=68.7% , the others are mainly polymer impurities. The decompression distillation vacuum degree is 200Pa (absolute pressure), 210 ℃ of tower still temperature, and tower top obtains the dehydrolinalool 157g that purity is 98.3% altogether. The vacuum degree was adjusted to 100 Pa, the temperature of the tower bottom was 280° C., and a total of 575 g of geranylacetonylenediol with a purity of 98.5% was obtained at the top of the tower, wherein the content of nerolidol was 0.33%, and the content of polymer impurities was 0.01%. The material obtained at the top of the tower is a by-product after pretreatment.
Embodiment 3
[0064] Example 3 Preparation of supported metal ruthenium catalyst I
[0065] Catalyst I preparation process is as follows: take by weighing 1.0g ruthenium trichloride solid and 0.1g zinc sulfate (as zinc modifier), 0.1g aluminum nitrate (aluminum modifier) are dissolved in 50g pure water, stir 60min, make fully dissolved. Add 5.0g of zirconium dioxide as a carrier and stir for 60 minutes to make it fully mixed. Add dropwise a 5% potassium borohydride aqueous solution to reduce it. With the dropwise addition of the potassium borohydride solution, the pH value of the reaction system changed. When the pH value rose from less than 2 to pH 4, the dropwise addition was stopped. The dropwise addition time was 0.5h, and the stirring reduction reaction was continued for 60min. After washing with pure water for 10 times, the supported metal ruthenium catalyst I was obtained, which was ready for use.
[0066] The mass percent content of metal ruthenium in the prepared supported met...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com