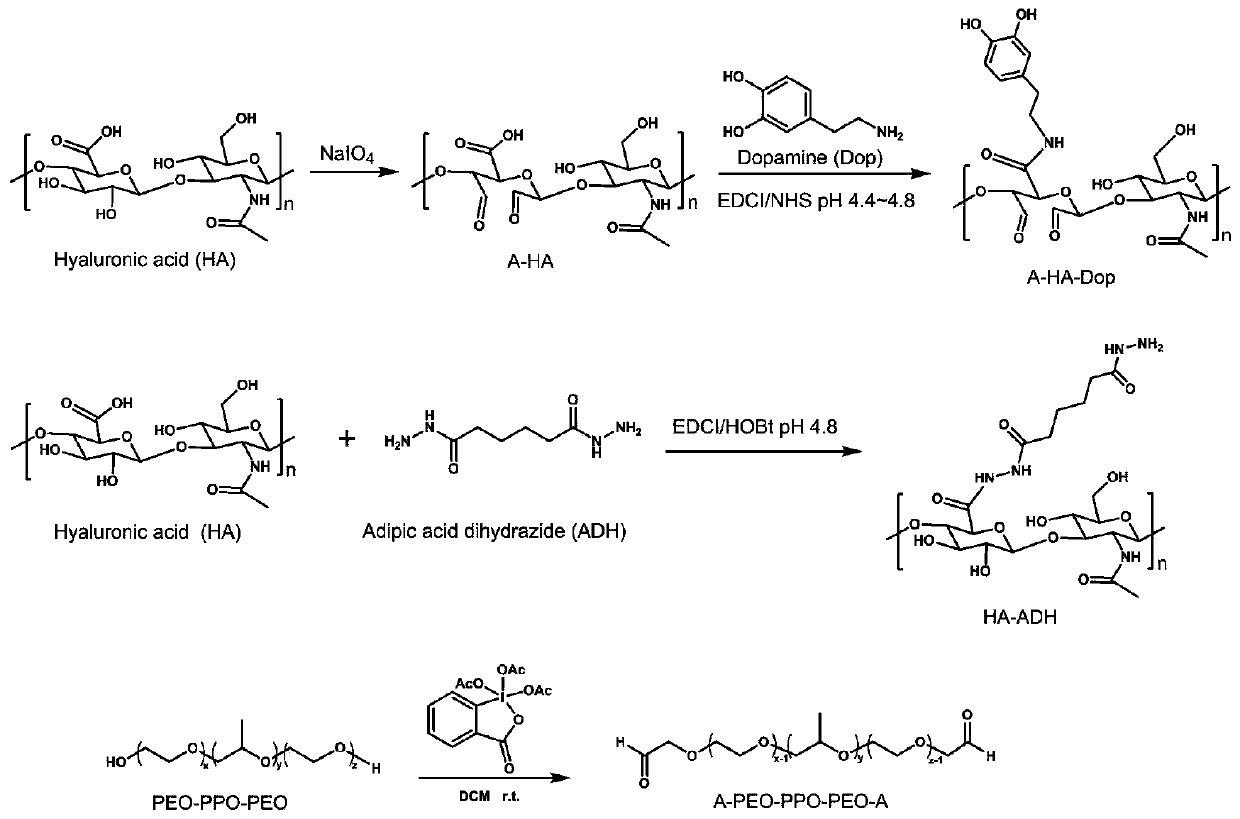
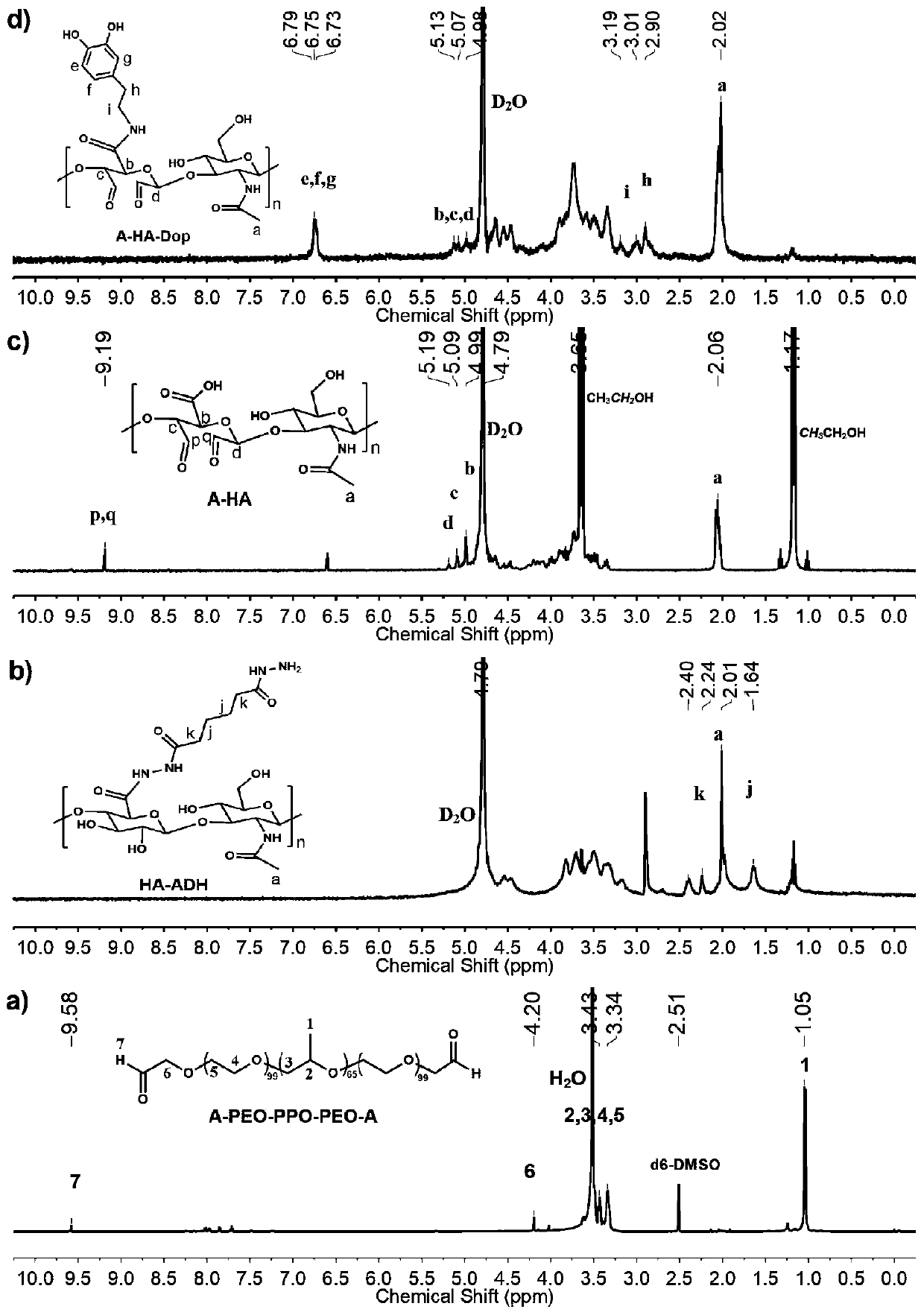
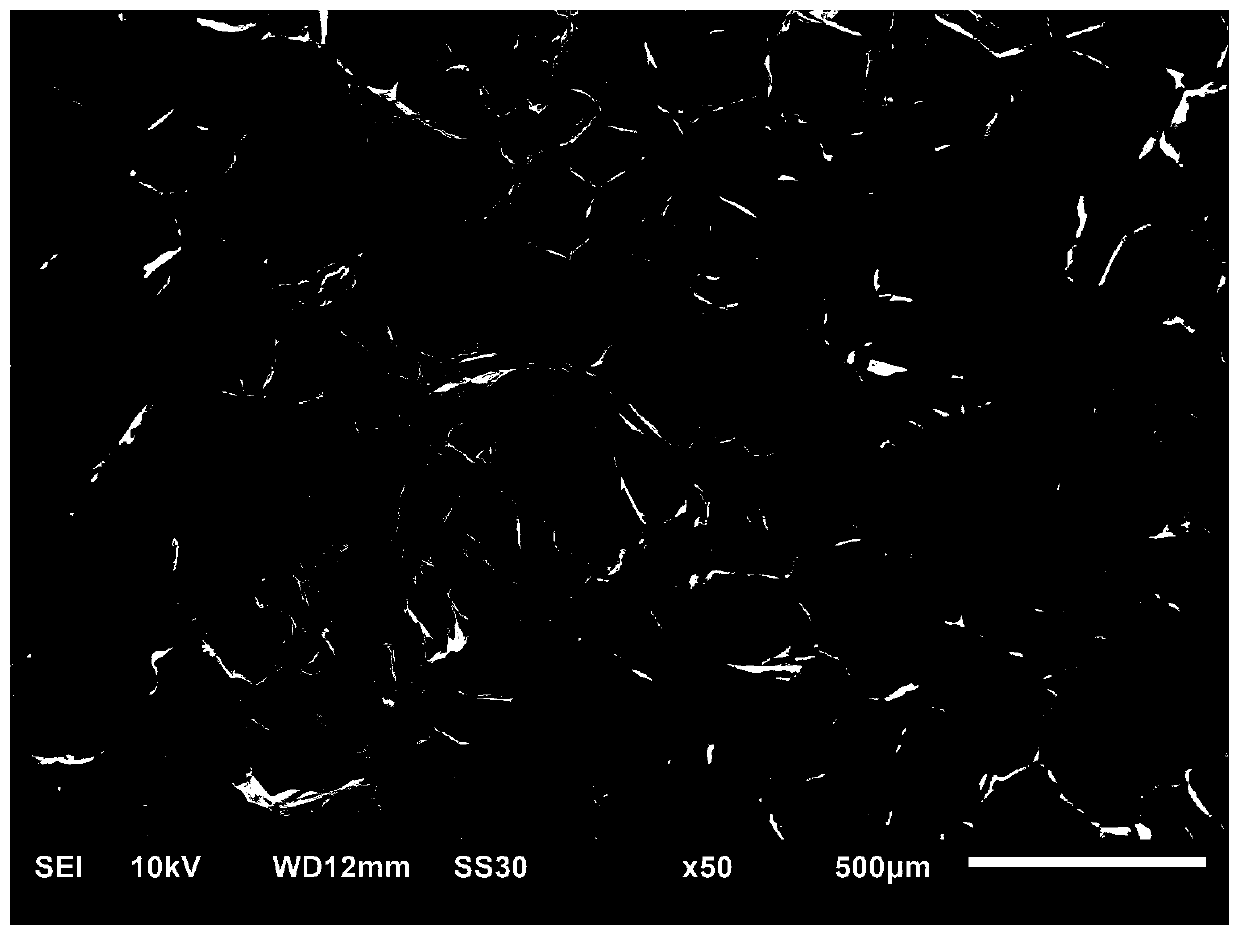
Injectable hydrogel as well as preparation method and application thereof
A technology for injecting water and hydrogel, which is applied in pharmaceutical formulations, medical preparations with non-active ingredients, and pharmaceutical sciences. It can solve problems such as weak mechanical strength, poor adhesion, and toxicity to surrounding tissues, and achieve biocompatibility Good sex and strength-enhancing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] (1) Preparation of aldehyde-terminated PEO-PPO-PEO triblock polymer (A-PEO-PPO-PEO-A): specific reaction parameters are shown in Table 1 below:
[0047] Table 1 Reaction parameter table for preparing A-PEO-PPO-PEO-A with different aldehyde modification degrees
[0048]
[0049] Dissolve PEO-PPO-PEO triblock polymer PF127 (0.5000g, 0.0390mmol) in 40mL of dichloromethane, add Dess-Martin Periodinane (0.0033g, 0.0078mmol), at 40°C Under reaction 12h. After the reaction was completed, after the reaction solution was cooled to room temperature, the reaction solution was rotary evaporated to viscous by evaporation under reduced pressure, then the viscous reaction solution was dropped into stirred n-hexane, stirred at room temperature for 4 hours, centrifuged (3000r, 5min) 3 to 5 times until the supernatant is clear, take the precipitate, put it in a vacuum drying oven and dry it overnight to obtain the aldehyde-terminated PEO-PPO-PEO triblock polymer (A-PEO-PPO-PEO-A), I...
Embodiment 2
[0066] (1) Preparation of aldehyde-terminated PEO-PPO-PEO triblock polymer (A-PEO-PPO-PEO-A):
[0067]Dissolve PEO-PPO-PEO triblock polymer PF127 (0.5000g, 0.0390mmol) in 40mL of dichloromethane, add Dess-Martin Periodinane (0.0156g, 0.0390mmol), at 40°C Under reaction 24h. After the reaction was completed, after the reaction solution was cooled to room temperature, the reaction solution was rotary evaporated to viscous by evaporation under reduced pressure, then the viscous reaction solution was dropped into stirred n-hexane, stirred at room temperature for 4 hours, centrifuged (3000r, 5min) 3 to 5 times until the supernatant is clear, take the precipitate, put it in a vacuum drying oven and dry it overnight to obtain the aldehyde-terminated PEO-PPO-PEO triblock polymer (A-PEO-PPO-PEO-A), It is in the form of white powder. Among them, the degree of oxidation of terminal hydroxyl groups at both ends of PF127 to aldehyde groups is about 58%.
[0068] (2) Preparation of hydra...
Embodiment 3
[0076] (1) Preparation of aldehyde-terminated PEO-PPO-PEO triblock polymer (A-PEO-PPO-PEO-A):
[0077] Dissolve PEO-PPO-PEO triblock polymer PF127 (0.5000g, 0.0390mmol) in 40mL of dichloromethane, add Dess-Martin Periodinane (0.0400g, 0.3000mmol), at 40°C Under reaction 24h. After the reaction was completed, after the reaction solution was cooled to room temperature, the reaction solution was rotary evaporated to viscous by evaporation under reduced pressure, then the viscous reaction solution was dropped into stirred n-hexane, stirred at room temperature for 4 hours, centrifuged (3000r, 5min) 3 to 5 times until the supernatant is clear, take the precipitate, put it in a vacuum drying oven and dry it overnight to obtain the aldehyde-terminated PEO-PPO-PEO triblock polymer (A-PEO-PPO-PEO-A), It is in the form of white powder. Among them, the degree of oxidation of terminal hydroxyl groups at both ends of PF127 to aldehyde groups is about 100%.
[0078] (2) Preparation of hyd...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| degree of grafting | aaaaa | aaaaa |
| degree of grafting | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com