Fluoro-substitution diphenylethane derivative and preparation method and application thereof
A technology of diphenylethane and derivatives, which is applied in the field of drug synthesis, can solve the problems of many by-products, tumor cell apoptosis, harsh reaction conditions, etc., and achieve the effect of inhibition, obvious effect, and strong anti-tumor activity in vitro
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
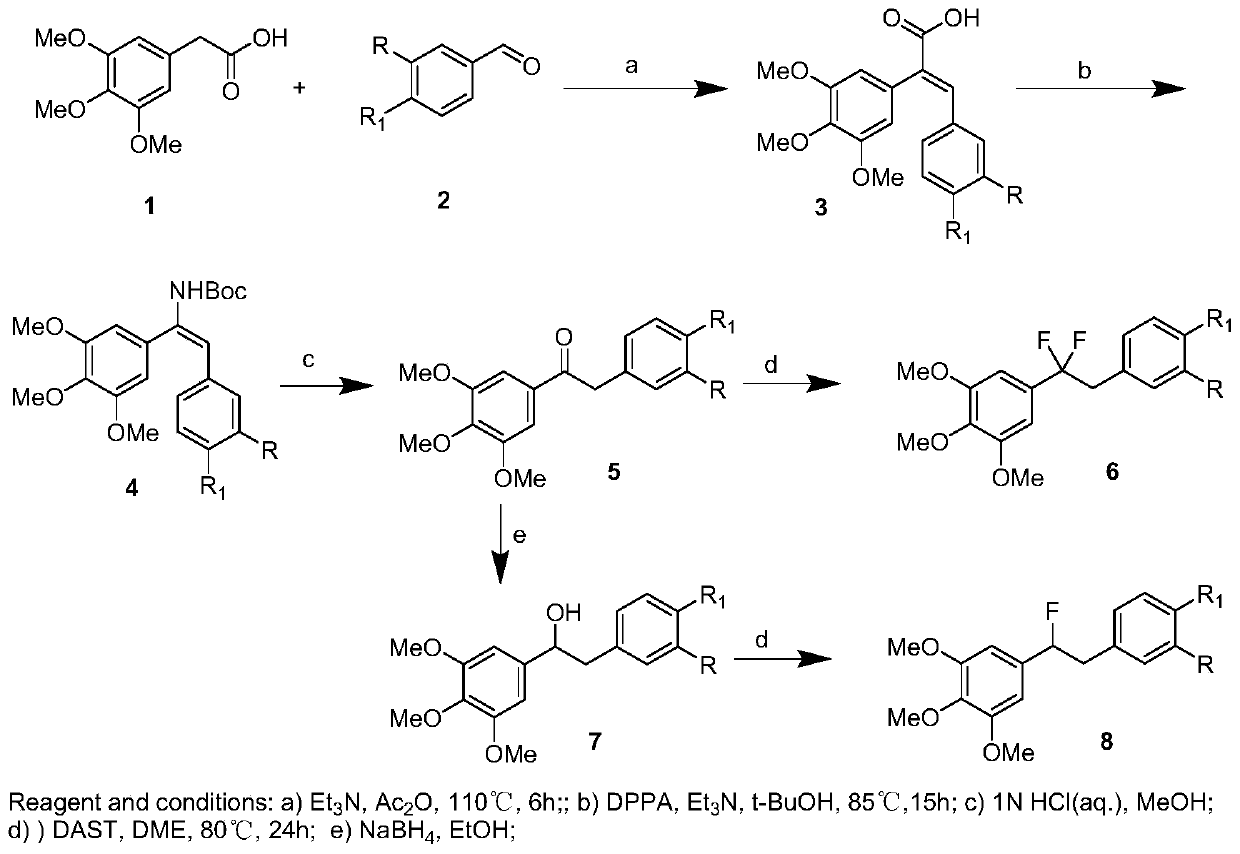
[0043] Synthesis of (E)-3-(4-methoxyphenyl)-2-(3,4,5-trimethoxyphenyl)acrylic acid.
[0044]3,4,5-trimethoxyphenylacetic acid (9.1g, 40mmol) and p-methoxybenzaldehyde (2a, 5.5g, 40mmol) were dissolved in 150mL of acetic anhydride, and triethylamine (8.1g, 80mmol), the mixture was heated at 110°C for 6h. After cooling, it was acidified with concentrated hydrochloric acid, poured into ice water, and stirred for 4 hours to obtain a light yellow solid, which was filtered, dissolved in 10% NaOH aqueous solution, washed with ethyl acetate to decolorize, the organic layer was separated, and the aqueous phase was retained. Hydrochloric acid was added to the aqueous phase until pH = 3-4. The precipitated solid was filtered and recrystallized from ethyl acetate to obtain 3a, 10.8g, yield 78.6%, white solid, mp: 185.6-186.0°C.
[0045] 1 H NMR (500MHz, CDCl 3 )δ7.88(s,1H),7.05(d,J=10.0Hz,2H),6.72(d,J=5.0Hz,2H),6.46(s,2H),3.89(s,3H),3.77( s,9H);
[0046] 13 C NMR (125MHz, CDCl 3 )...
Embodiment 2
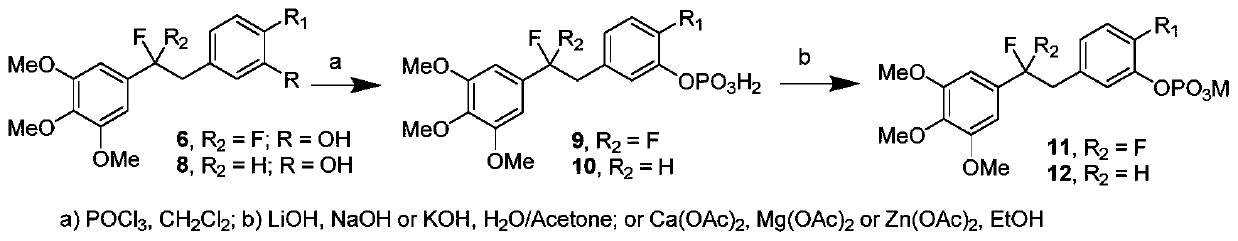
[0067] Preparation of 5-(1,1-difluoro-2-(4-methoxyphenyl)ethyl)-1,2,3-trimethoxybenzene (6a)
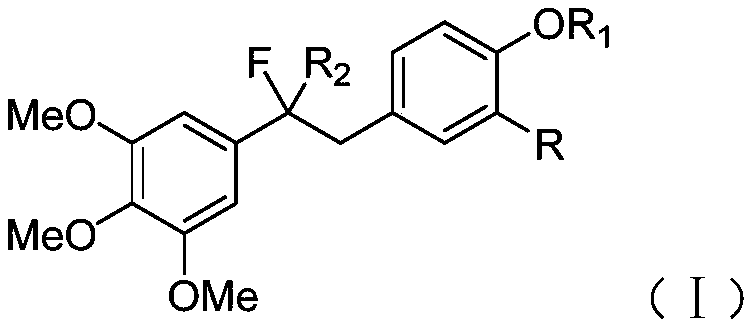
[0068] It is one of the fluorine-substituted diphenylethane derivatives.
[0069] Put 5a (5mmol), DAST (15mL) and ethylene glycol dimethyl ether (5mL) into a pressure-resistant glass-sealed tube reactor, seal it, heat to 80°C, and stir for 24h. Cool to room temperature, open the bottle cap, pour the reaction solution into ice water, extract with ethyl acetate, combine the organic phases, dry with anhydrous sodium sulfate, filter, spin to dryness and concentrate, and purify by column chromatography to obtain 6a (0.7g, yield 43.2%), yellow oil.
[0070] 1 H NMR (500MHz, CDCl 3 )δ7.02(d, J=10.0Hz, 2H), 6.79(d, J=5.0Hz, 2H), 6.47(s, 2H), 3.84(s, 3H), 3.77(s, 9H), 3.31( t, J HF =15.5Hz,2H); 19 F NMR (470MHz, CDCl 3 )δ-94.42(s);
[0071] 13 C NMR (125MHz, CDCl 3 )δ158.96, 152.94(x2), 138.93, 132.25(t, J CF = 25.63Hz), 131.66(x2), 124.78(t, J CF=10Hz), 121.92(t, J CF =242.5Hz),...
Embodiment 3
[0074] Preparation of 5-(2,2-difluoro-2-(3,4,5-trimethoxyphenyl)ethyl)-2-methoxyphenol (6d).
[0075] It is one of the fluorine-substituted diphenylethane derivatives.
[0076] Put 5c (5mmol), DAST (15mL) and ethylene glycol dimethyl ether (5mL) into a pressure-resistant glass-sealed tube reactor, seal it, heat to 80°C, and stir for 24h. After cooling to room temperature, the reaction solution was poured into ice water, extracted with ethyl acetate, the organic phases were combined, dried over anhydrous sodium sulfate, filtered, concentrated by spinning to dryness, and purified by column chromatography to obtain 6c, a yellow oil. used directly in the next step.
[0077] Dissolve 6c in ethanol, add 10% palladium on carbon, and hydrogenate at normal pressure to obtain 6d (0.7g, two-step yield 40.1%), white solid, mp:98.3-98.9℃.
[0078] 1 H NMR (500MHz, CDCl 3 )δ6.85-6.71(m,2H),6.56-6.36(m,3H),5.61(s,1H), 3.84(s,3H),3.79-3.75(m,9H),3.29(t,J HF =17.5,2H);
[0079] 19 F NMR...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com