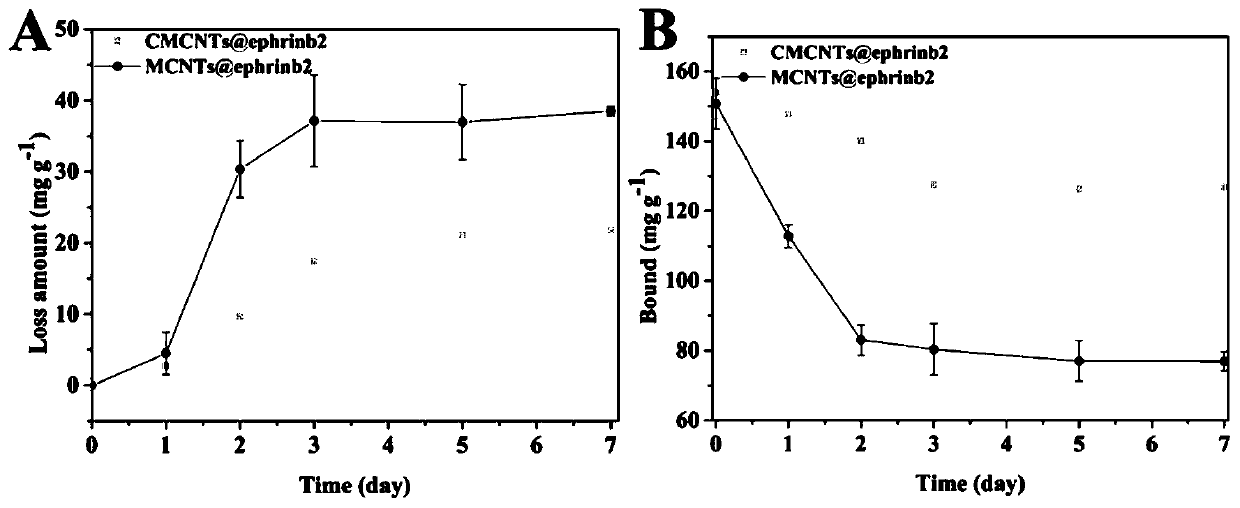
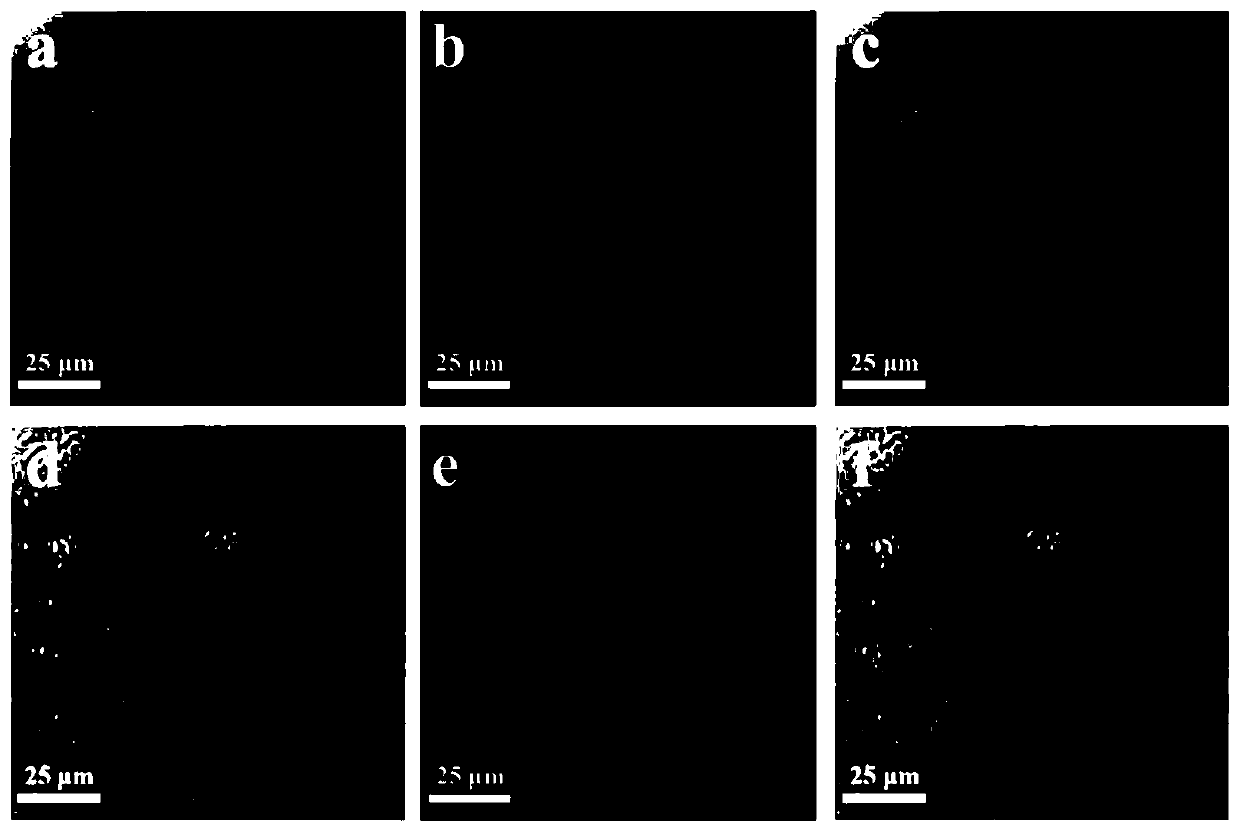
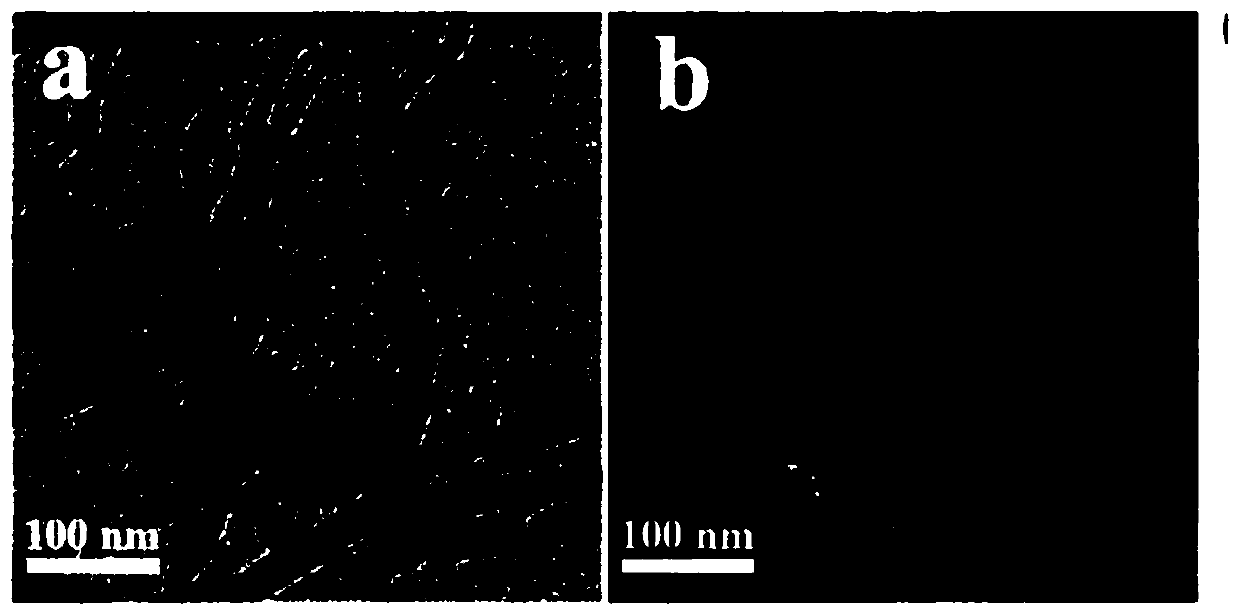
Cell membrane magnetic carbon nanotube drug screening material and preparation method and application
A technology of magnetic carbon nanotubes and cell membranes, which is applied in the field of bionic materials, can solve problems such as membrane coating shedding and instability, and achieve the effects of improving bonding strength, stability, and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] 1. Materials and methods
[0043] 1. Experimental materials
[0044] CNTs-COOH (Jiangsu Xianfeng Nano Material Technology Co., Ltd.); ferrous sulfate heptahydrate (FeSO 4 ·7H 2 O), ferric chloride hexahydrate (FeCl 3 ·6H 2 O), ammonia (25%) (Guangdong Guanghua Science and Technology Co., Ltd.); RIPA lysis buffer (WB009, Xi'an Hert Biotechnology Co., Ltd.). DiI dye (Shanghai Biyuntian Biotechnology Co., Ltd.). Neoaconitine, deltaline, 13-dehydroxyaconitine (Baoji Chenguang Biotechnology Co., Ltd.). Gefitinib, nitrendipine, verapamil, tamsulosin (Nanjing Ange Pharmaceutical Chemical Co., Ltd.). Aconitum (Shaanxi Xi'an Pharmaceutical Market).
[0045] 2. Preparation of CMCNTs@ephrinb2
[0046] 2.1 Preparation of CMCNTs
[0047] MCNTs were prepared by one-step co-precipitation method, the specific process is: 0.06g FeCl 3 ·6H 2 O and 0.03gFeSO 4 ·7H 2 O was ultrasonically dissolved in 80 mL of distilled water, and 0.1 g of CNTs was ultrasonically dispersed and ...
Embodiment 2
[0088] Select the ephrinb2 / HEK293 cells that have been attached to the wall of the bottle, and count the cells. When the number of cells is not less than 10 7 Digest with trypsin solution to obtain cell suspension; centrifuge the cell suspension to precipitate ephrinb2 / HEK293 cells, add Tris-HCl buffer solution to ephrinb2 / HEK293 cells, then ultrasonically break the cells, and finally centrifuge to obtain The supernatant was centrifuged, and the resulting pellet was the cell membrane. The cell membrane was resuspended with physiological saline to obtain a cell membrane suspension.
[0089] FeCl 3 ·6H 2 O and FeSO 4 ·7H 2 O was dissolved in distilled water, and then CNTs were added to adjust the pH value to 9. Under the protection of nitrogen, the mixture was stirred at 70°C for 70 min and magnetically separated to obtain MCNTs. Among them, FeCl 3 ·6H 2 O, FeSO 4 ·7H 2 The ratio of O to CNTs is 0.05g:0.05g:0.08g.
[0090] Disperse CMCNTs in water to form an aqueous MC...
Embodiment 3
[0093] Select the ephrinb2 / HEK293 cells that have been attached to the wall of the bottle, and count the cells. When the number of cells is not less than 10 7 Digest with trypsin solution to obtain cell suspension; centrifuge the cell suspension to precipitate ephrinb2 / HEK293 cells, add Tris-HCl buffer solution to ephrinb2 / HEK293 cells, then ultrasonically break the cells, and finally centrifuge to obtain The supernatant was centrifuged, and the resulting pellet was the cell membrane. The cell membrane was resuspended with physiological saline to obtain a cell membrane suspension.
[0094] FeCl 3 ·6H 2 O and FeSO 4 ·7H 2 O was dissolved in distilled water, and then CNTs were added to adjust the pH value to 11. Under the protection of nitrogen, the mixture was stirred at 90°C for 50 min, and magnetically separated to obtain MCNTs. Among them, FeCl 3 ·6H 2 O, FeSO 4 ·7H 2 The ratio of O to CNTs is 0.10g:0.01g:0.12g.
[0095]Disperse CMCNTs in water to form an aqueous M...
PUM
| Property | Measurement | Unit |
|---|---|---|
| mass | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com