Method for preparing calcium fluoride from phosphorous ore-associated fluorine resources
A calcium fluoride and resource technology, applied in the field of phosphorus chemical industry, can solve the problems of high safety, environmental protection risks, limited application scope, inability to apply, etc., and achieve the effects of widening the range of raw materials, good dissolution effect and low loss rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
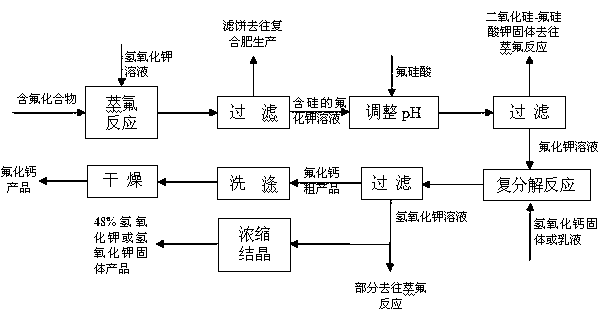
[0042] Add 220g of potassium fluorosilicate prepared in advance with fluorosilicic acid to 220g of water to make a slurry, then add 490g of 48% (mass concentration, the same below) potassium hydroxide solution, stir and react at 90°C for 60min, and then filter to obtain Silica filter cake and solution, the filter cake is washed to obtain white carbon black products; the filtrate is added with fluorosilicic acid, a by-product of phosphate fertilizer, to adjust the pH to 8, and filtered to obtain refined potassium fluoride solution and filter cake (for washing) , the filter cake is returned to the fluorine extraction reaction, the lotion is combined with the potassium fluoride solution; 402 g of calcium hydroxide solid is added to the combined potassium fluoride solution, and after 20 minutes of reaction, filter, wash, and dry at 105 ° C to obtain calcium fluoride product, the filtrate is concentrated to 48% concentration and partly returns to the fluorine extraction reaction, an...
Embodiment 2
[0045] Add 220g of crystals to 220g of water to make a slurry, then add 463g of 48% potassium hydroxide solution, stir and react at 95°C for 60min, then filter to obtain a filter cake and solution, and the filter cake is washed as a raw material for compound fertilizer preparation; the filtrate Then add fluosilicic acid, a by-product of phosphate fertilizer, to adjust the pH to 7, and obtain refined potassium fluoride solution and filter cake (for washing) after filtration. Add 175g of calcium hydroxide solid to the potassium chloride solution, react for 30 minutes, filter, wash, and dry at 105°C to obtain the calcium fluoride product. The filtrate obtained 48% potassium hydroxide product after defluorination.
[0046] Obtain calcium fluoride 180g in this process, productive rate 97.5%, calcium fluoride content 98.1%, silicon dioxide content 0.08%, calcium carbonate content 0.97%, sulfur content 0.04%, P content 0.05%, arsenic is not detected, Organic matter was not detected....
Embodiment 3
[0048] Add 220g of sediment to 150g of water to make a slurry, then add 297g of 48% potassium hydroxide solution, stir and react at 80°C for 60 minutes, then filter to obtain filter cake and solution, and wash the filter cake as raw material for compound fertilizer preparation The filtrate is added to the phosphate fertilizer by-product fluosilicic acid to adjust the pH to 7, and after filtration, the refined potassium fluoride solution and filter cake (for washing) are obtained, and the filter cake is returned to the fluorine extraction reaction, and the washing solution is combined with the potassium fluoride solution; Add 1:1 (mass ratio of calcium hydroxide to water) calcium hydroxide emulsion 220g to the potassium fluoride solution, react for 30 minutes, filter, wash, dry at 105°C to obtain the calcium fluoride product, and concentrate the filtrate to 48% After the concentration, part returns to the fluorine extraction reaction, and the remaining filtrate is defluorinated,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com