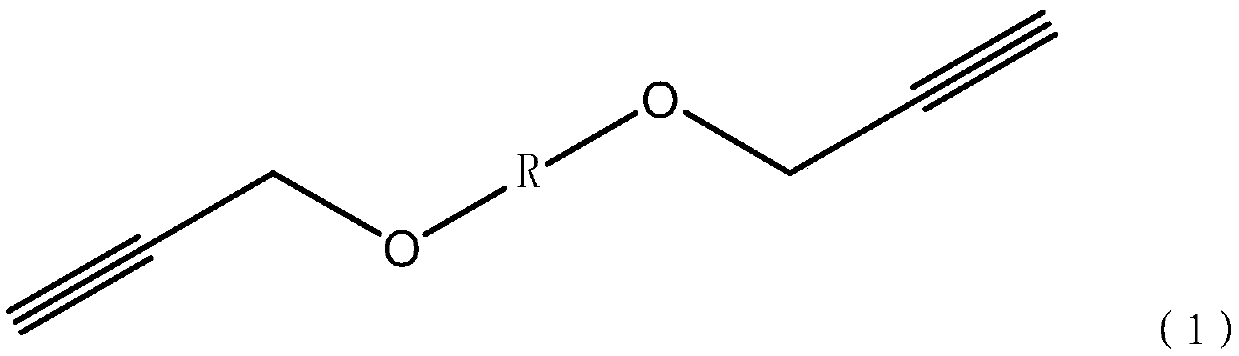
Nonisocyanate-cured azide polyether binder system and propellant
A non-isocyanate and azide polyether technology, applied in explosives and other fields, can solve the problems of unreasonable rigid structure network crosslinking structure, poor mechanical properties, low mechanical properties, etc., achieve stable and controllable charge quality, and solve thermal problems. Stress problem, the effect of excellent mechanical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0054] In an optional embodiment, the preparation method of the alkynyl-terminated polyether comprises the following steps:
[0055] (1): Dissolve the hydroxyl-terminated polyether in an organic solvent, and add an alkali metal hydride into the hydroxyl-terminated polyether solution under the protection of an inert gas, wherein the molar percentage range of the hydroxyl-terminated polyether and the alkali metal hydride is 1 :2~1:3;
[0056] Wherein, the organic solvent can be tetrahydrofuran, acetone, ethanol, etc., preferably tetrahydrofuran; the alkali metal hydride can be sodium hydride, potassium hydride, lithium hydride, etc., preferably sodium hydride;
[0057] (2): React the mixed solution obtained in step (1) at 20-40°C for 3h-5h;
[0058] (3): The system after the reaction in step (2) is cooled to 0-10°C, and after the temperature is balanced, propyne bromide is added dropwise in proportion, wherein the molar percentage of propyne bromide and alkali metal hydride is ...
Embodiment 1
[0074] (1) Preparation of alkynyl-terminated polyether:
[0075] (a) Dissolving PEG (Mn=700) in tetrahydrofuran to obtain a solution, adding sodium hydride to the solution in a nitrogen atmosphere in proportion, wherein the molar percentage of PEG and sodium hydride is 1:2;
[0076] (b) Move the mixed solution obtained in step (a) into a constant temperature reaction bath at 30° C., and the reaction time is 4 hours;
[0077] (c) The reactant obtained in step (b) is moved into an ice-water bath, and after the temperature is balanced, propyne bromide is added dropwise in proportion, and the molar ratio of propyne bromide to sodium hydride is 1:1.5;
[0078] (d) Seal the reactant obtained in step (c) with nitrogen gas, and move the reaction system into a temperature environment of 20°C, and continue to react at 20°C for 48h;
[0079] (e) filtering the reactant obtained in step (d), and evaporating the THF solvent to dryness to obtain the crude product of the alkynyl-terminated p...
Embodiment 2
[0091] (1) Preparation of alkynyl-terminated polyether:
[0092] The preparation method is basically the same as in Example 1, except that the raw material used in step (a) is QJPEG (Mn=3500), and the final alkynyl-terminated polyether obtained is QJPEG (Mn=3500);
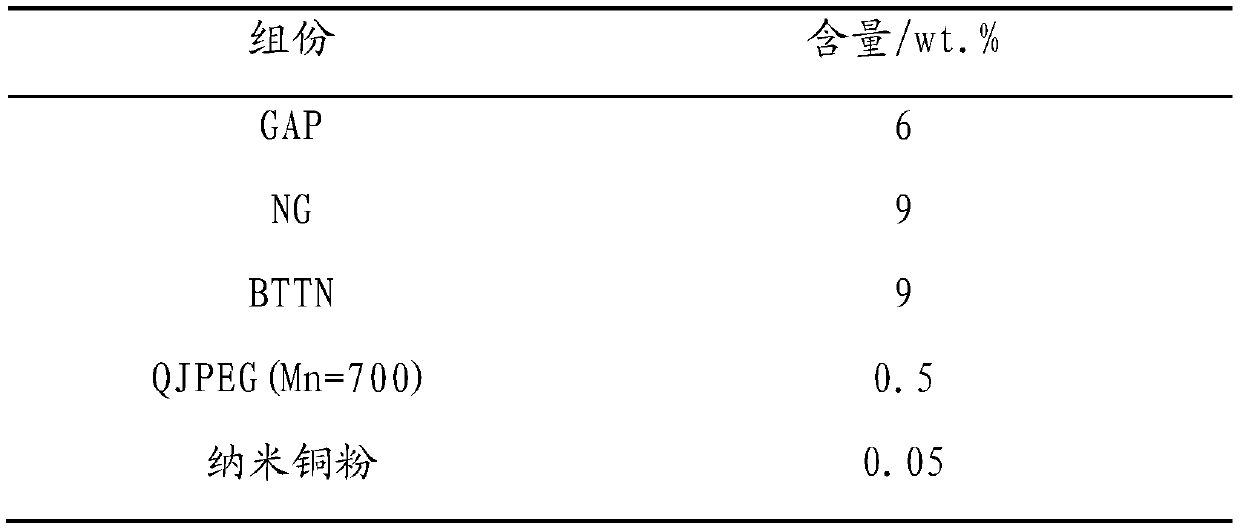
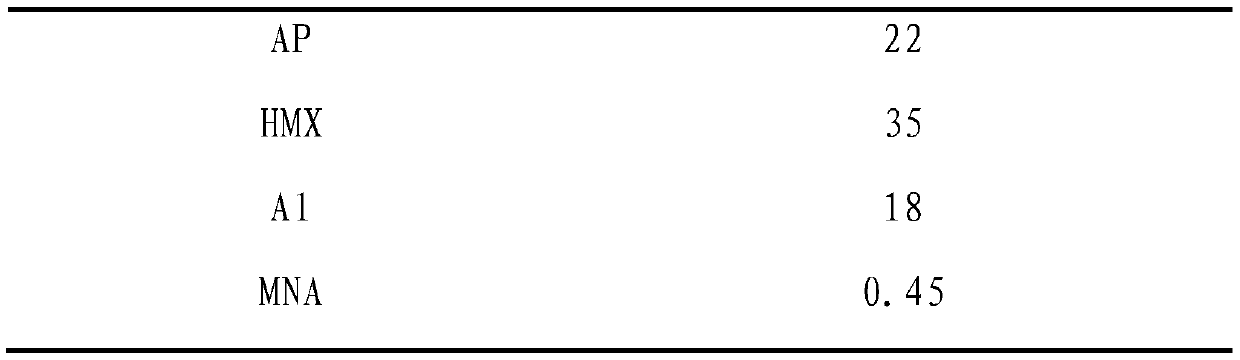
[0093] (2) Propellant formula composition (mass percent %):
[0094]
[0095] (3) Propellant performance:
[0096] Theoretical standard specific impulse: 2664.6N.s / kg (271.9s)
[0097] Mechanical properties: Maximum tensile strength σ at 20°C m =0.40MPa, maximum elongation ε m =143.2%
[0098] Maximum elongation at 70℃ε m=35.9%
[0099] -40℃maximum elongationε m = 39.6%
PUM
| Property | Measurement | Unit |
|---|---|---|
| Tensile strength | aaaaa | aaaaa |
| Tensile strength | aaaaa | aaaaa |
| Tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com