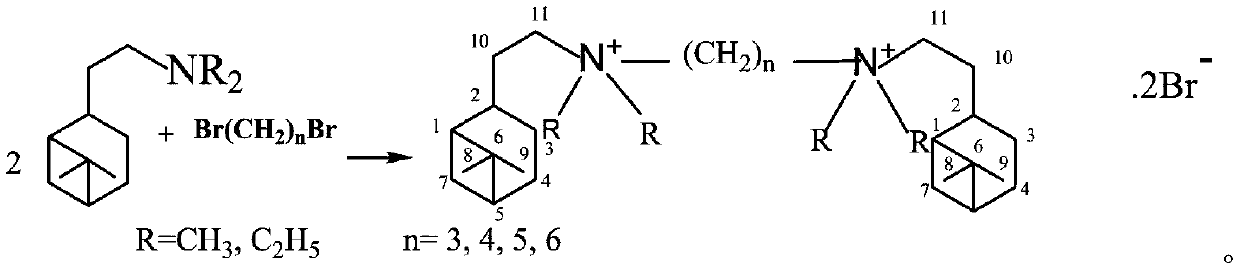
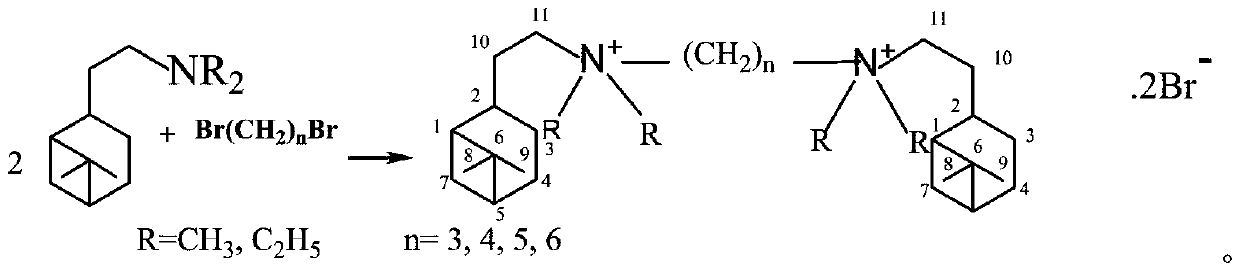
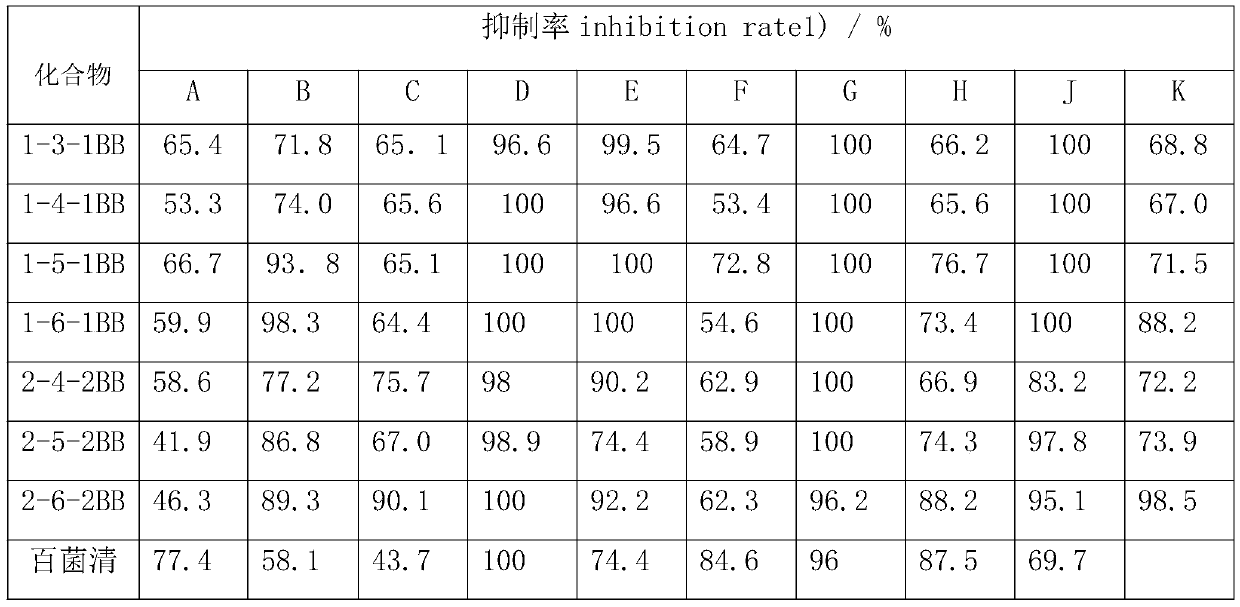
Synthesis method of hydrogenated nopyl-containing symmetric genini quaternary ammonium iron and antibacterial application
A technology for hydrogenating nobyl and gemini quaternary ammonium, applied in the fields of application, botany equipment and methods, fungicides, etc., can solve the problems of large pesticide residues, loss of agricultural, forestry and animal husbandry production, environmental pollution, etc., and achieve product yield and The effect of high purity, mild conditions and easy control, and simple equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] In a 150mL ground-necked Erlenmeyer flask, add 55mmoL of nobyldimethylamine, 25mmoL of 1,3-dibromopropane, 50mL of methanol / ethanol / isopropanol / methyl acetate / ethyl acetate / acetone / butanone / Put acetonitrile into a stirring bar, install a spherical condenser, place it on a magnetic heating stirrer for stirring and reflux, stop heating after 24 hours, cool to 10°C, and obtain crystals, filter the crystals, and suction filter with cold petroleum ether to Dry and vacuum dry to obtain trimethylene-1,3-bis(hydrogenated dimethyl ammonium bromide) (1-3-1BB) finished product. White fine columnar crystals, the yield is 81%, m.p.245.7-247.3℃. NMR, δH (CDCl3): 3.748 (4H, t, J = 8Hz, 212-CH3), 3.543 (4H, m, 211-CH2), 3.326 (12H, s, 4α-CH3), 2.359 (2H, m, 2 2-CH),2.292(2H,m,13-CH2),2.018~1.698[6H,m,2(7-CH,10-CH2,5-CH,1-CH,4-CH2,3-CH )], 1.434(2H,m,25-CH),1.150(6H,s,29-CH3),0.988(6H,s,28-CH3),0.830(2H,d,J=9.6Hz,27-CH ); δC(CDCl3): 38.585(C-1), 46.286(C-2), 22.328(C-3), 26.268(C-4)...
Embodiment 2
[0020] In a 150mL ground-necked Erlenmeyer flask, add 55mmoL of nobyldimethylamine, 25mmoL of 1,4-dibromobutane, 50mL of methanol / ethanol / isopropanol / methyl acetate / ethyl acetate / acetone / butanone / acetonitrile, put in a stirring bar, install a spherical condenser, place on a magnetic heating stirrer for stirring and reflux, stop heating after 24 hours, cool to 10°C to obtain crystals, filter the crystals, and suction filter with cold petroleum ether To dryness, vacuum-dry to obtain tetramethylene-1,4-bis(hydrogenated dimethyl ammonium bromide) (1-4-1BB) finished product. White fine columnar crystals, the yield is 87%, m.p.263.7-265.5℃. NMR,δH(CDCl3):3.817(4H,m,212-CH2),3.045(4H,m,211-CH2),3.240(12H,s,4α-CH3),2.288(2H,m,22-CH2) ,2.041~1.765[20H,m,2(7-CH,10-CH2,5-CH,1-CH,4-CH2,3-CH),213-CH2], 1.427(2H,m,23-CH ),1.147(6H,s,9-CH3),0.975(6H,s,28-CH3),0.821(2H,d,J=9.6Hz, 27-CH); δC(CDCl3):37.782(C-1 ), 45.339(C-2), 21.363(C-3), 25.80(C-4), 40.859(C-5), 38.047(C-6), 29.430(C-7), ...
Embodiment 3
[0022] In a 150mL ground-necked Erlenmeyer flask, add 55mmoL of nobyldimethylamine, 25mmoL of 1,5-dibromopentane, 50mL of methanol / ethanol / isopropanol / methyl acetate / ethyl acetate / acetone / butanone / acetonitrile, put in a stirring bar, install a spherical condenser, place on a magnetic heating stirrer for stirring and reflux, stop heating after 24 hours, cool to 10°C to obtain crystals, filter the crystals, and suction filter with cold petroleum ether To dryness, vacuum-dry to obtain pentamethylene-1,5-bis(hydrogenated dimethyl ammonium bromide) (1-5-1BB) finished product. White crystals, yield 85%, m.p.239.1-241.7°C. NMR, δH(CDCl3): 3.365~3.280(8H, m, 211-CH2, 212-CH2), 3.051(12H, s, 4α-CH3), 2.329(2H, m, 22-CH2), 1.934~1.826[ 12H, m,2(7-CH,10-CH2,5-CH,3-CH)],1.719(8H,m,24-CH2,213-CH2),1.475(2H,m,2 3-CH) ,1.289(2H,m,14-CH2),1.817(6H,s,29-CH3),1.036(6H,s,28-CH3),0.859(2H,d,J=9.6Hz,27-CH); δC(CDCl3): 37.924(C-1), 45.420(C-2), 21.731(C-3), 25.908(C-4), 40.977(C-5), 38.116(C-6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com