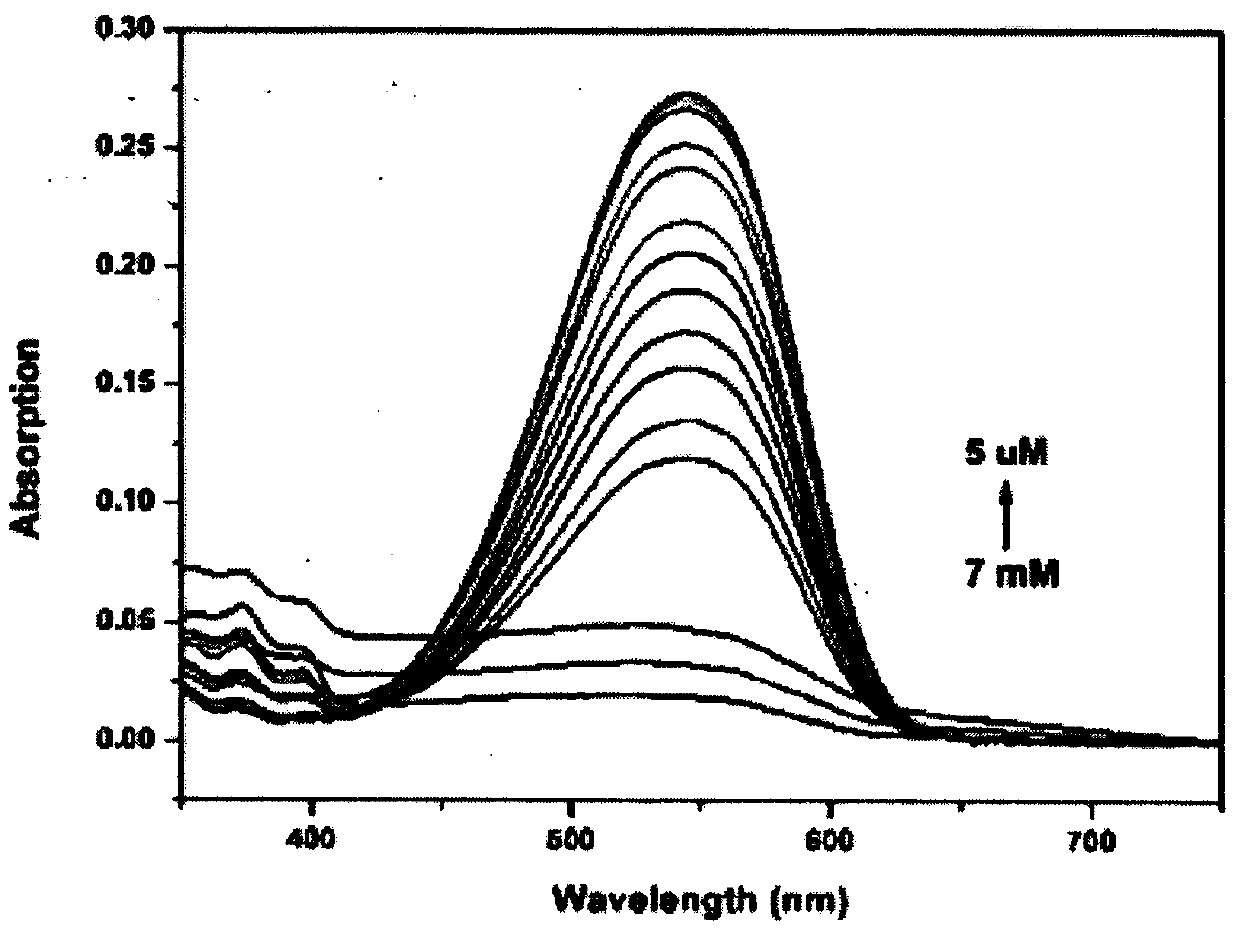
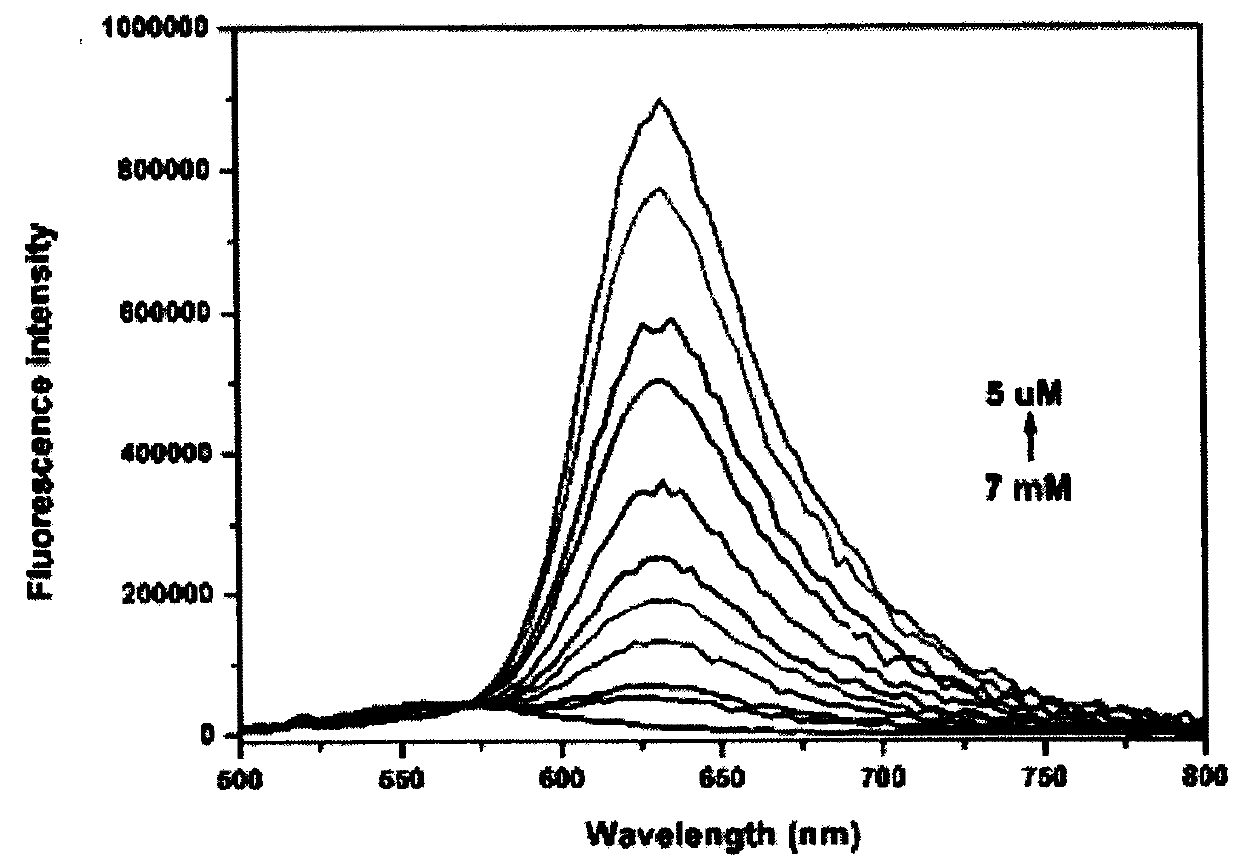
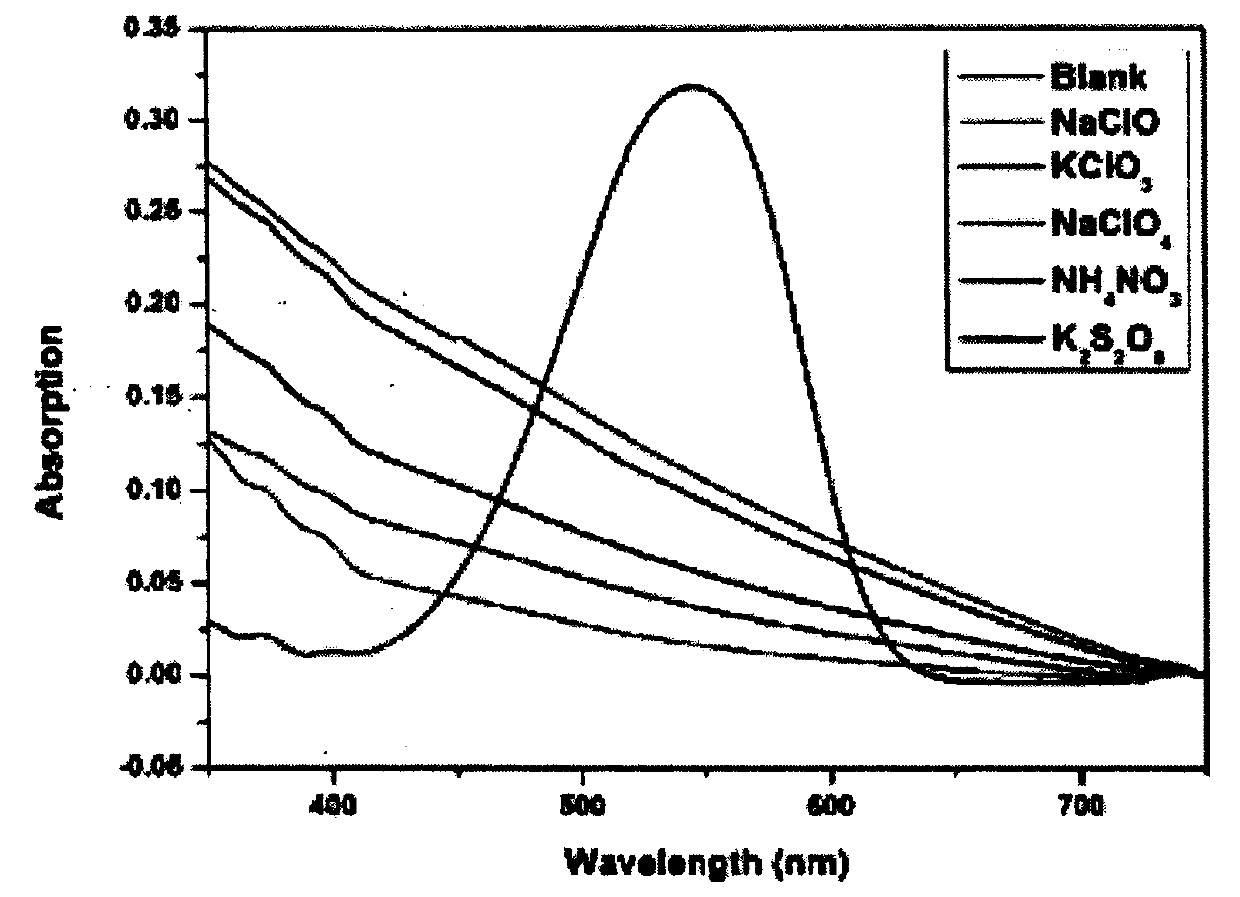
Colorimetric-fluorescent probe for detecting hypochlorite radical, preparation method and application thereof
A technology for the detection of hypochlorous acid and fluorescent probes, applied in the field of environmental and non-standard explosives detection, can solve the problems of low quantum yield, low molar absorptivity, detection limit, etc., and achieve the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] a. Dissolve 5mmol N-phenyldiethanolamine in 50mL organic solvent ether, add 20mmol organic base as triethylamine, add 20mmol acryloyl chloride at 0°C, react at room temperature for 12h, extract, wash with deionized water until neutral and anhydrous After drying over magnesium sulfate, suction filtration and spin-drying, 4.6 mmol of intermediate product 1 (phenylazadiyl) bis(ethane-2,1-diyl) diacrylate was obtained, with a yield of 92%;
[0036] b. Drop 4.6 mmol of phosphorus oxychloride into 23 mmol of N,N-dimethylformamide at 0°C, react for 2 hours, then drop into 4.6 mmol of intermediate product 1 obtained in step a, raise the temperature to 75°C, and react for 12 hours After cooling to room temperature, add the organic solvent diethyl ether, the temperature is 0°C, take 30mmol of anhydrous sodium acetate and dissolve it in 50mL of deionized water to prepare an aqueous solution of sodium acetate, then drop the prepared aqueous solution of sodium acetate into the reacta...
Embodiment 2
[0039] a. Dissolve 5mmol N-phenyldiethanolamine in 50mL organic solvent chloroform, add 10mmol organic base as piperidine, add 10mmol acryloyl chloride at 0°C, react for 12h, extract, wash with deionized water until neutral, anhydrous sulfuric acid After magnesium drying, suction filtration and spin-drying, 4.5 mmol of intermediate product 1 (phenylazadiyl) bis(ethane-2,1-diyl) diacrylate was obtained, with a yield of 90%;
[0040] b. Add 4.5mmol of phosphorus oxychloride dropwise into 22.5mmol of N,N-dimethylformamide at 0°C, react for 2 hours, then drop into 4.5mmol of the intermediate product 1 obtained in step a, raise the temperature to 75°C, and react After 12 hours, cool to room temperature, add organic solvent chloroform, temperature 0°C, take 30mmol anhydrous sodium acetate and dissolve it in 50mL deionized water to prepare an aqueous solution of sodium acetate, then drop the prepared aqueous solution of sodium acetate into the reactant, and react at room temperature f...
Embodiment 3
[0043] a. Dissolve 5mmol N-phenyldiethanolamine in 50mL organic solvent tetrahydrofuran, add 15mmol organic base as pyridine, add 15mmol acryloyl chloride at 0°C, react for 12h, extract, wash with deionized water until neutral, and dry with anhydrous magnesium sulfate , suction filtration and spin-drying, 4.7 mmol of intermediate product 1 (phenylazadiyl) bis(ethane-2,1-diyl) diacrylate was obtained, with a yield of 94%;
[0044] b. Drop 4.7mmol of phosphorus oxychloride into 23.5mmol of N,N-dimethylformamide at 0°C, react for 2 hours, then drop into 4.7mmol of the intermediate product 1 obtained in step a, raise the temperature to 75°C, and react After 12 hours, cool to room temperature, add the organic solvent tetrahydrofuran, the temperature is 0°C, take 30mmol of anhydrous sodium acetate and dissolve it in 50mL of deionized water to prepare an aqueous solution of sodium acetate, then drop the prepared aqueous solution of sodium acetate into the reactant, and react at room t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com