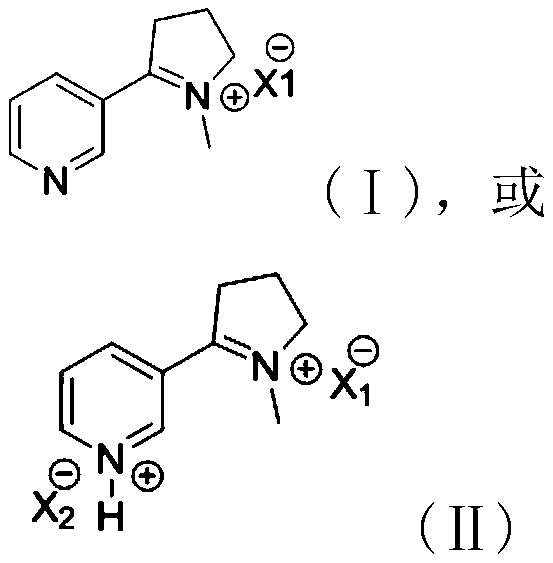
Imine salt derivative, preparation method thereof and preparation method of nicotine
A technology of imide salts and derivatives is applied in the field of preparation of organic synthesis, can solve the problems of incomplete reaction of intermediate pyrrolidinol, unsuitable for industrial large-scale production, expensive starting materials, etc., and achieves high synthesis rate and synthetic Mild process conditions and high purity results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
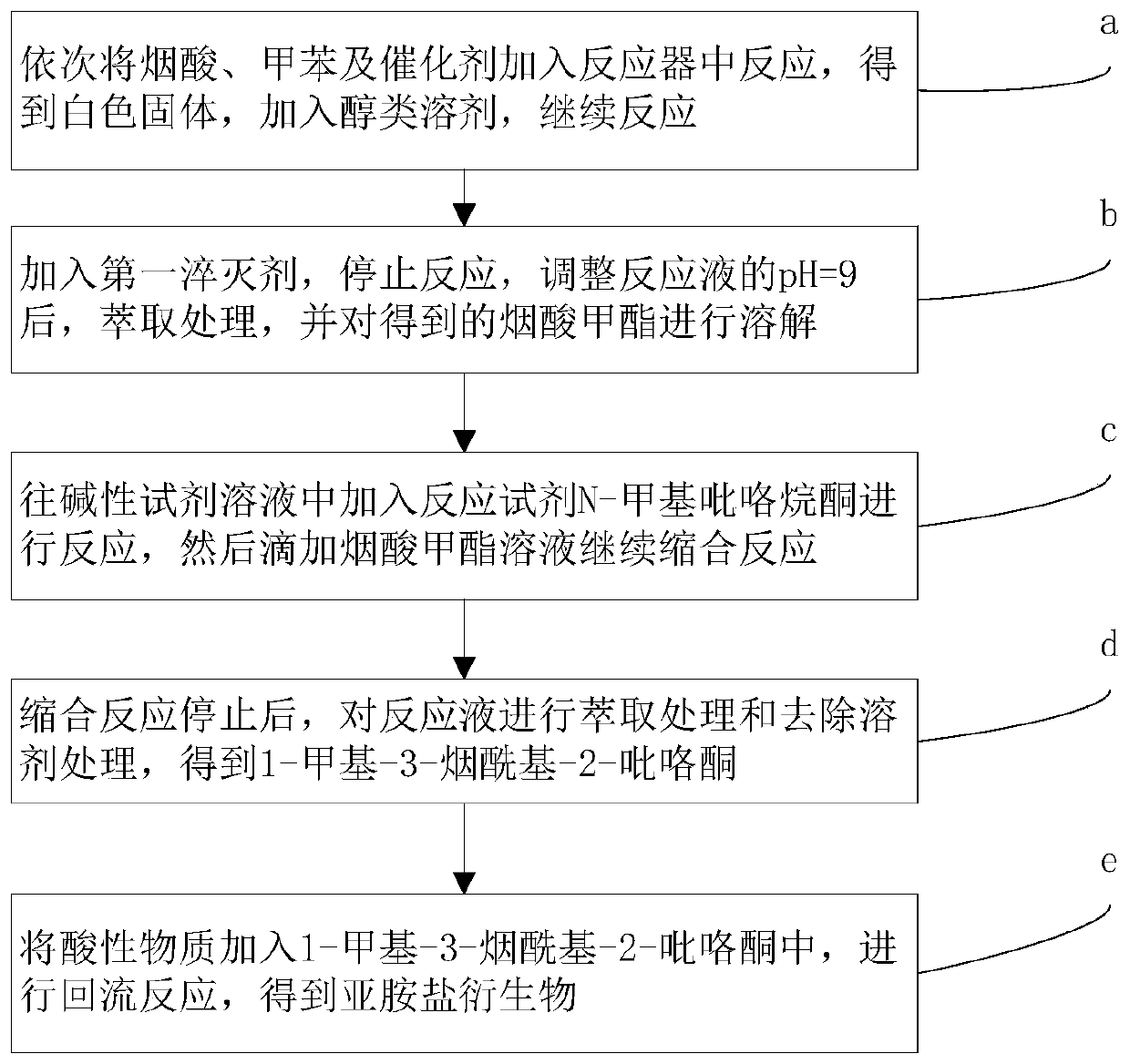
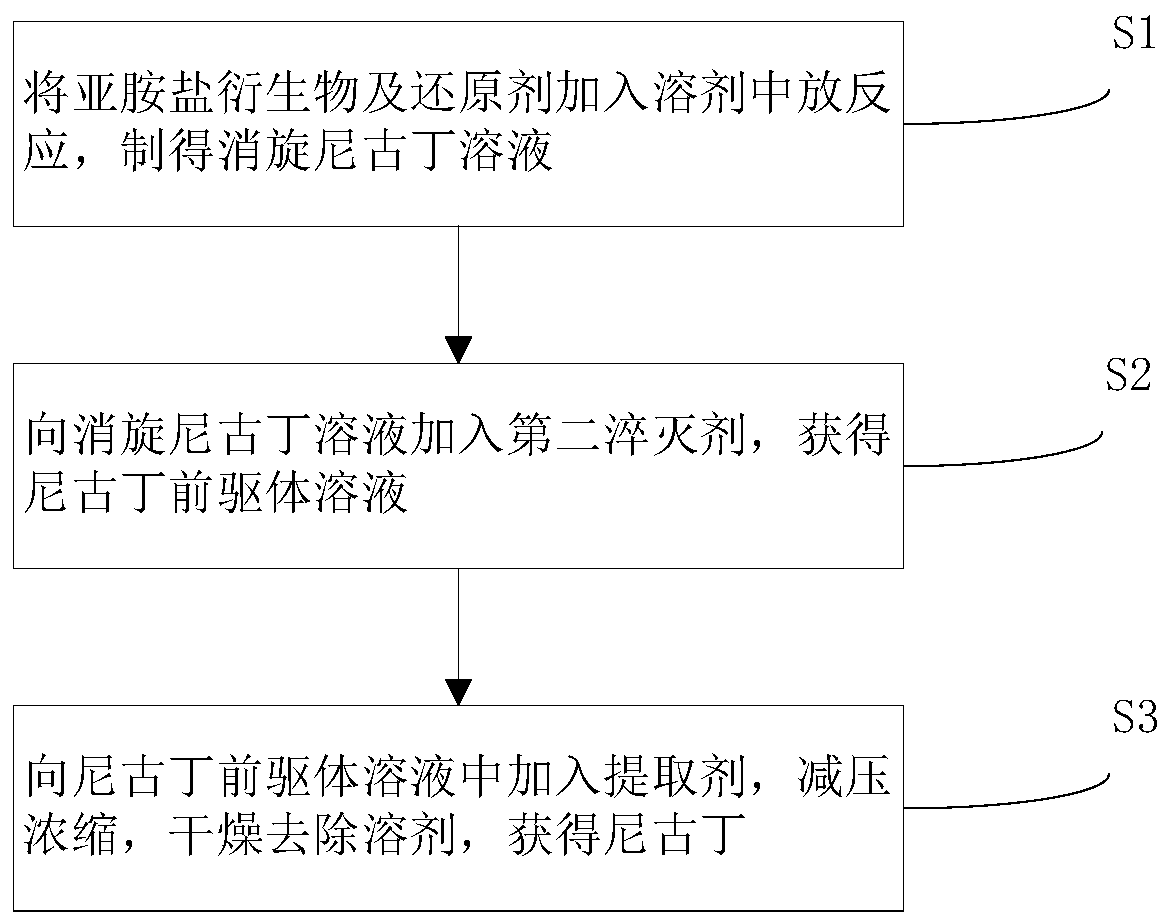
[0048] Such as figure 1 Shown, above-mentioned iminium salt derivative, its preparation method process step is as follows:
[0049] a. Add nicotinic acid, toluene and catalyst into the reactor in turn, and react under stirring and heating conditions to obtain a mixed solution; the mixed solution is concentrated under reduced pressure to remove the solvent to obtain a white solid; Down stirring, continue reaction.
[0050] In this step, the molar ratio of nicotinic acid to the catalyst is 1:5, and the catalyst is thionyl chloride or oxalyl chloride, preferably thionyl chloride; toluene is used as a solvent to dissolve nicotinic acid and the catalyst, which is beneficial to the reaction . Since nicotinic acid and the catalyst are insoluble in toluene at room temperature, they need to be heated to 50-80° C. (preferably 70° C.) during the dissolution process and stirred while heating. During the reaction process, in order to ensure constant temperature heating, the oil temperat...
Embodiment 1
[0090] Synthesis of imide derivatives, i.e. intermediate hydrochloride compounds
[0091]
[0092] Add 1.0g (8.1mmoL, 1eq) of nicotinic acid, 3mL of toluene, and 3mL of thionyl chloride (41.3mmoL, 5eq) in sequence, stir at room temperature to insoluble, heat the oil at 70°C for 0.5h to dissolve, and obtain a colorless transparent solution; After heating and reacting for 3 h, TLC showed that the raw material was completely reacted. Concentrate the above reaction solution under reduced pressure at 60°C to obtain a white solid, add 6mL of methanol, stir at room temperature (30°C) for 0.5h, adjust the pH to 9 with 5% aqueous sodium carbonate (the test paper shows dark green), and add 30mL of acetic acid Extracted twice with ethyl ester and concentrated under reduced pressure to obtain 0.67 g of methyl nicotinate.
[0093] Add NaH (2.4g) into 20mL toluene, stir at room temperature (25°C) for 10min, add N-methylpyrrolidone (3.65g) and stir at room temperature (25°C) for 15min, n...
Embodiment 2
[0097] The difference with Example 1 is: the first quenching agent is selected potassium hydroxide aqueous solution; Alcohol extractant is selected ethyl acetate; The alkaline reagent that condensation reaction adopts is sodium ethylate; Sulfuric acid was used in the example.
[0098] Add concentrated sulfuric acid to 1-methyl-3-nicotinoyl-2-pyrrolidone (2.0g) aqueous solution, exotherm, the reaction solution is light yellow, reflux at 110°C for 24h, cool down to 10°C, and distill off water under reduced pressure , adding acetone to recrystallize to obtain the sulfate salt of the intermediate (1.6g).
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com