Fluorescent quantitative PCR on-site rapid detection kit for African swine fever virus
A technology for African swine fever virus and detection kits, which is applied in the field of on-site rapid detection kits for African swine fever virus fluorescence quantitative PCR, can solve the problems of many steps in the extraction process, large differences in test results, and many types of reagents, etc., and achieve reduction Time and operation steps, suitable for transportation and storage, and the effect of saving detection time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
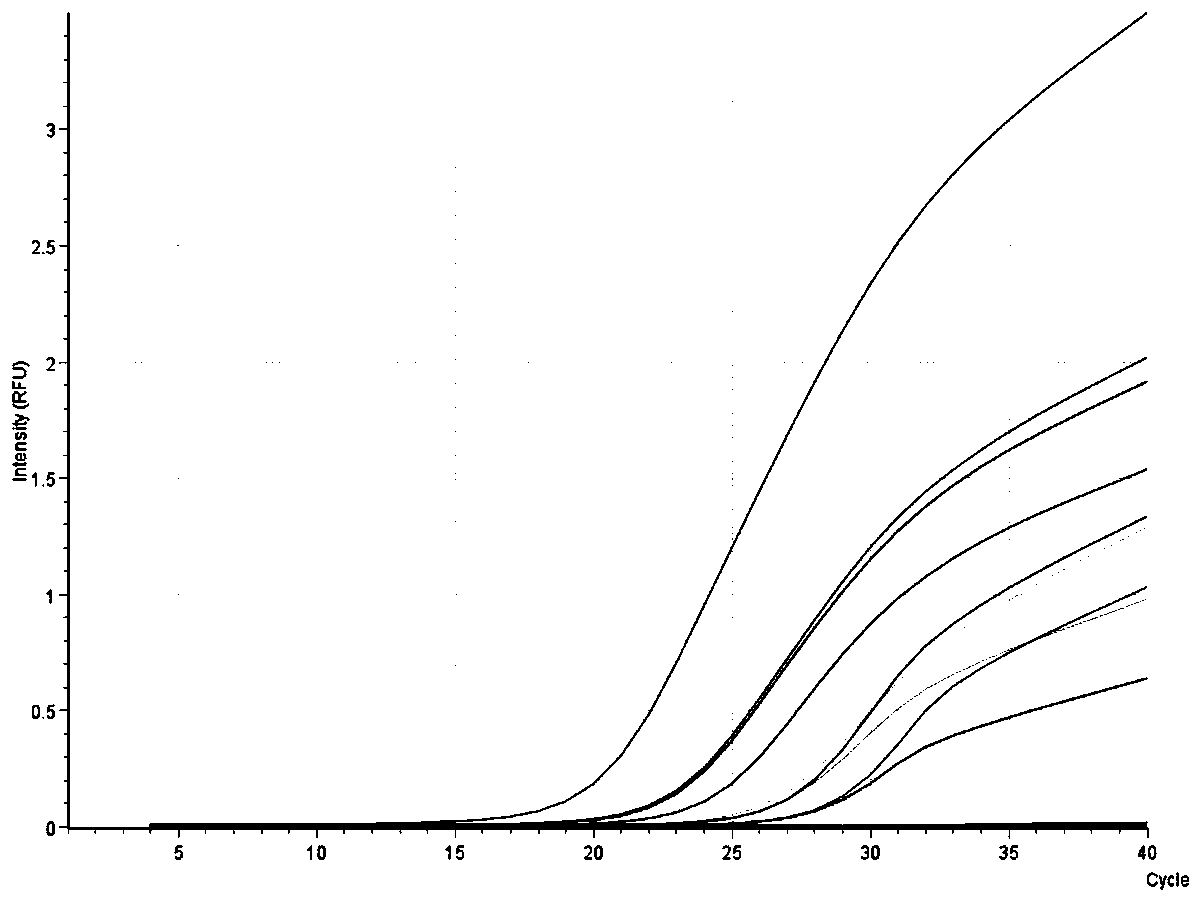
[0026] Embodiment 1: Detection of clinical whole blood samples
[0027] A fluorescent quantitative PCR detection kit for African swine fever virus, said kit comprising: buffer B1, buffer B2, PCR reaction solution, positive control and negative control;
[0028] The buffer B1: guanidine isothiocyanate 1-6M, sodium chloride 0.1-3M, ethylenediaminetetraacetic acid 0.01-0.5M, adjust the pH value to 8.5-12.7;
[0029] The components of the buffer B2 are: ammonium acetate 0.1-1.0M, Tris-Cl 0.01-1M, and the pH value is adjusted to 7.5-8.5.
[0030] The PCR reaction solution: composed of 2×Premix Ex Taq TM Composed of enzyme mixture, ASFV-specific upstream and downstream primers and fluorescent probes, Tli RNaseH is added to the enzyme mixture; Tli RNaseH is a heat-resistant RNaseH, which can be well eliminated when DNA is used as a template for PCR reactions The primers and probe sequences of the obstacles that inhibit the PCR reaction are as follows:
[0031] Upstream primer: 5'-...
Embodiment 2
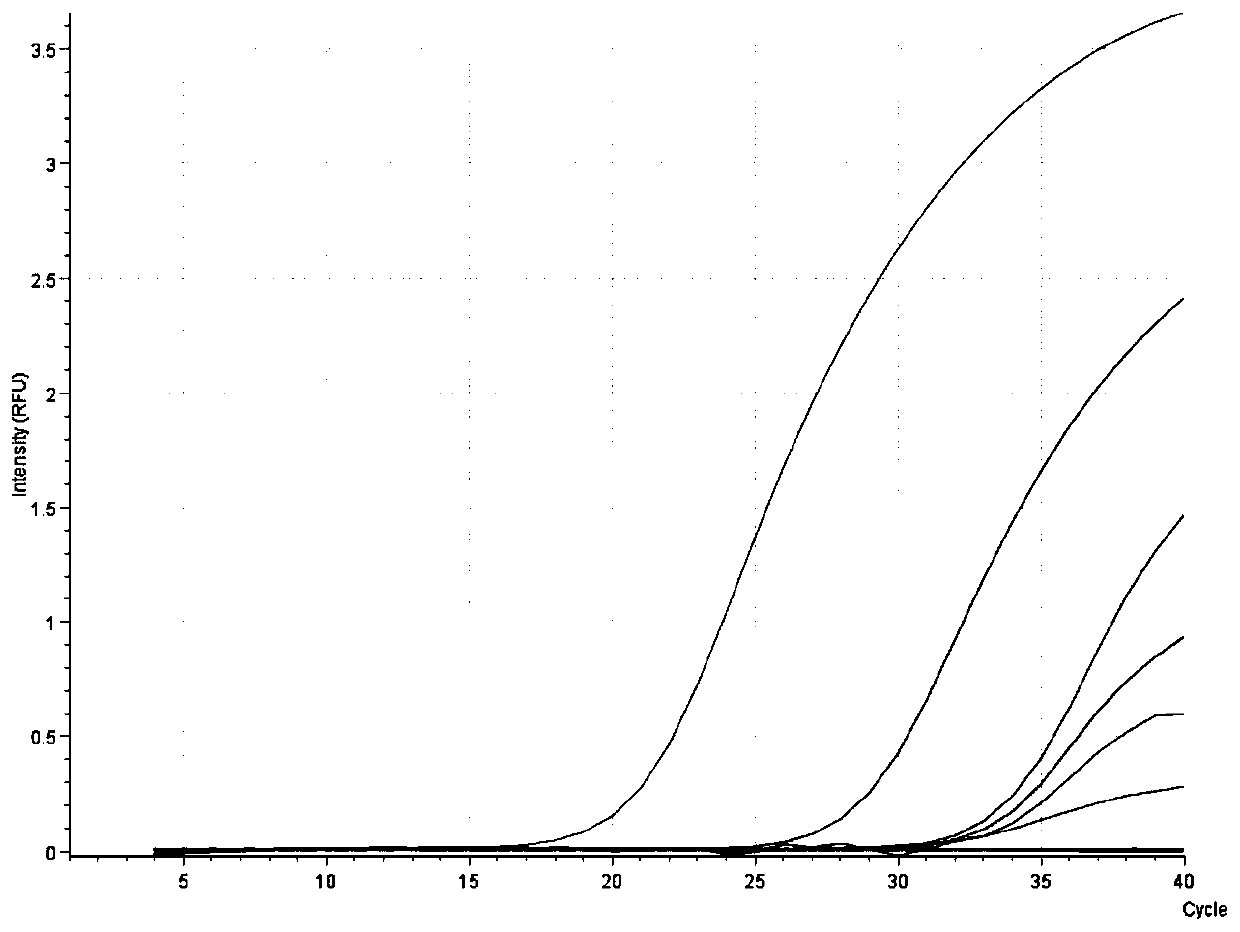
[0052] Embodiment 2: Detection of clinical tissue samples
[0053] The kit used in this example is the same as Example 1.
[0054] 1) Pretreatment of tissue samples
[0055] Collect 3 samples of spleen, 3 lymph nodes, and 4 muscle tissue samples, weigh 0.1-0.2g- (soy bean size) for each sample, add 10 times the volume of about 1-2ml normal saline (0.9% sodium chloride), Make a tissue homogenate, centrifuge at 10,000 rpm for 1 minute, and take the supernatant for the next step of nucleic acid extraction.
[0056] 2) Acquisition of sample DNA virus nucleic acid:
[0057] (1) Buffer B1 Lysis: First take a 1.5ml centrifuge tube and add 100 μL of buffer B1, take 10 samples of tissue homogenate that have been processed in the above steps, add 20 μL of each sample to buffer B1 and mix well, vortex at room temperature Rotate and shake to mix 3-5 times, lyse at room temperature for 3 minutes.
[0058] (2) Buffer B2 to terminate the lysis: add 100 μL of buffer B2 to the above mixtur...
Embodiment 3
[0069] Embodiment 3: The impact of different DNA virus genome extraction methods on ASFV detection
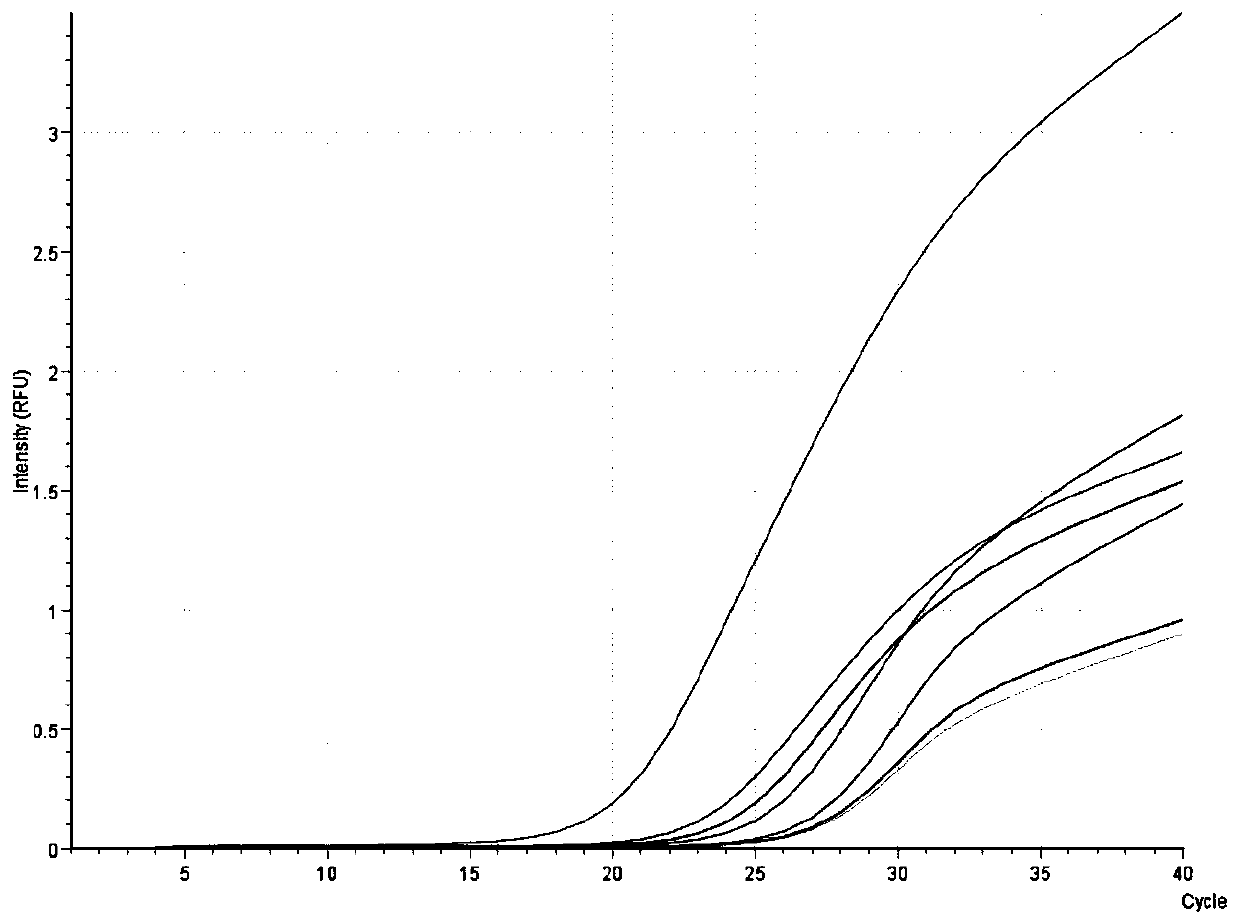
[0070] There are many types of DNA extraction kits currently on the market, such as column extraction method, magnetic bead method, etc. In this embodiment, the ASFV nucleic acid extracted by the common column extraction method and the magnetic bead method and the corresponding ASFV fluorescence quantitative PCR method are compared with the ASFV fluorescence quantitative PCR rapid detection kit based on nucleic acid extraction-free direct amplification developed by the present invention. To explore the influence of different DNA virus nucleic acid extraction methods on ASFV detection. In this example, two ASFV clinically positive serum samples were selected as samples to be tested, and after DNA was extracted by three methods, fluorescent PCR reaction was carried out. The detection steps are as follows:
[0071] 1. Extraction of DNA from samples
[0072] Method 1. Extraction...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com