Preparation method of Fe-OMS-2 catalyst and application of Fe-OMS-2 catalyst in degradation of organic pollutants
An organic pollutant, fe-oms-2 technology, applied in the direction of water pollutants, molecular sieve catalysts, chemical instruments and methods, etc., can solve the harsh conditions of photoactivated persulfate technology and high energy consumption of thermally activated persulfate technology problems, to achieve good catalytic degradation effect, excellent catalytic effect, and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] In a 250ml round bottom flask add MnSO 4 (8.8g), Fe(NO 3 ) 3 (0.6375g), then add 100ml of water, stir at room temperature for 10min, then continue to add 3ml of concentrated nitric acid and 5.89g of potassium permanganate (dissolved in 30ml of water) mixed solution, reflux at 100°C for 1d, then filter and wash to obtain Black powder, then dry the black powder at 120°C for 8h, after drying, calcined at 350°C for 2h to obtain the product.
[0038] figure 1 It is the characteristic scanning electron microscope picture of the Fe-OMS-2 catalyst of embodiment 1. The stick-shaped substance on the picture is Fe-OMS-2 catalyst.
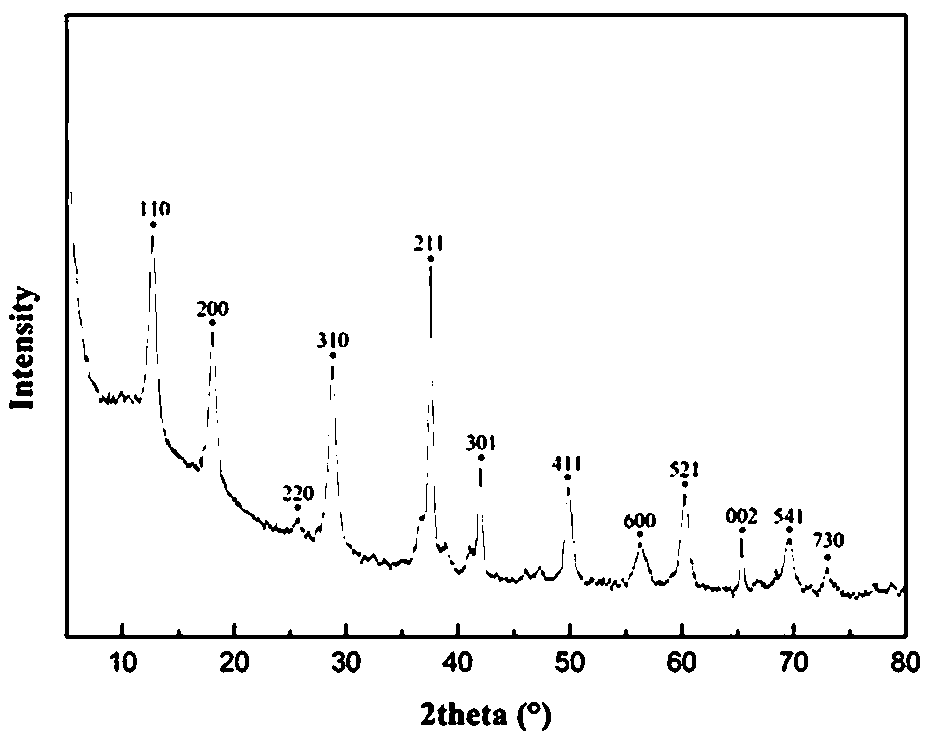
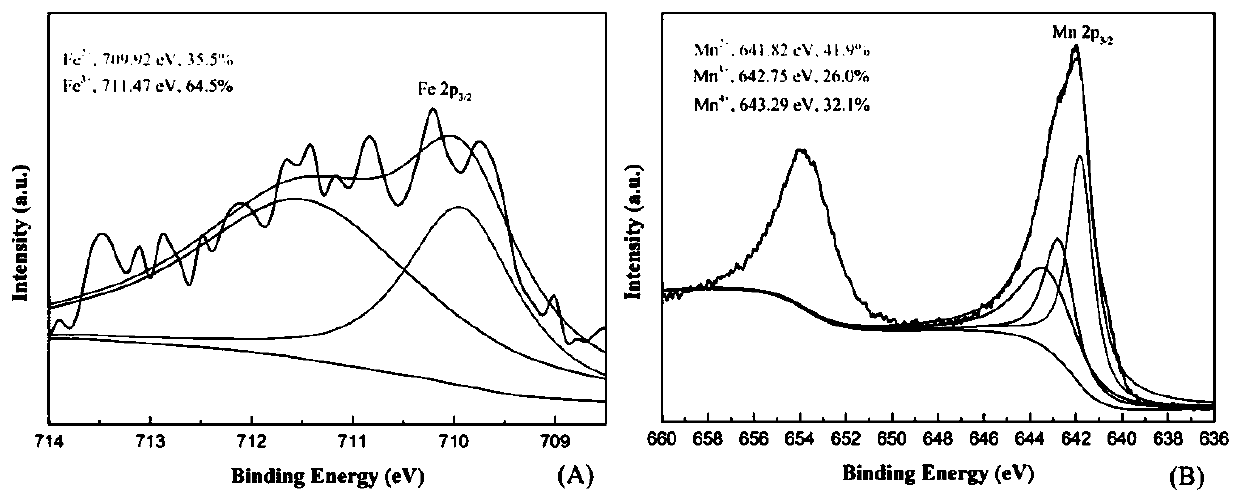
[0039] figure 2 , 3 Be the XRD figure and the XPS figure of the Fe-OMS-2 catalyst of embodiment 1, can find out from the XPS figure that Fe-OMS-2 catalyst contains Fe 2+ , Fe 3+ , Mn 2+ , Mn 3+ , Mn 4+ , where Fe 2+ The content is 35.5%, Fe 3+ The content is 64.5%, Mn 2+ The content is 41.9%, Mn 3+ The content is 26.0%, Mn 4+ The content ...
Embodiment 2
[0041] The application of Fe-OMS-2 prepared by the invention in catalyzing PMS to generate sulfate radicals to degrade Acid Orange 7.
[0042] The reaction mechanism of Fe-OMS-2 catalyzing PMS to generate sulfate radicals to degrade Acid Orange 7, that is, in the presence of PMS, the low-valence Fe in Fe-OMS-2 2+ , Mn 2+ or Mn 3+ is oxidized to Fe 3+ , Mn 4+ At the same time, it is accompanied by the generation of strong oxidizing sulfate radicals and hydroxyl radicals, thereby degrading Acid Orange 7.
[0043] The steps of described Fe-OMS-2 in catalyzing PMS to produce sulfate radical degradation Acid Orange 7 reaction are as follows:
[0044] Step 1: Acid Orange 7 solution (1ml 2.5×10 -4 mol / l) into the cuvette, add PMS solution (1ml 2.5×10 -3 mol / l);
[0045] Step 2: Test and record the peak absorption of Acid Orange 7 at this time;
[0046] Step 3: Quickly add the Fe-OMS-2 catalyst into the cuvette, and measure the peak shape of the ultraviolet-visible light absor...
Embodiment 3
[0050] The application of Fe-OMS-2 prepared by the invention in catalyzing PMS to produce sulfate radicals to degrade Methylene Blue.
[0051] The reaction mechanism of Fe-OMS-2 catalyzing PMS to generate sulfate radicals to degrade Methylene Blue, that is, in the presence of PMS, the low-valence Fe in Fe-OMS-2 2+ , Mn 2+ or Mn 3+ is oxidized to Fe 3+ , Mn 4+ At the same time, it is accompanied by the generation of strong oxidizing sulfate radicals and hydroxyl radicals, thereby degrading Methylene Blue. Described Fe-OMS-2 is in the step that catalysis PMS produces sulfate radical degradation Methylene Blue reaction as follows:
[0052] Step 1: Methylene Blue solution (1ml 0.75×10 -4 mol / l) into the cuvette, add PMS solution (1ml 0.75×10 -3 mol / l);
[0053] Step 2: Test and record the absorption peak of Methylene Blue at this time;
[0054] Step 3: Quickly add the Fe-OMS-2 catalyst into the cuvette, and measure the peak shape of the Methylene Blue UV-Vis absorption spe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com