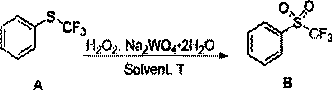Trifluoroalkyl sulfone compound preparation method
A technology of trifluoroalkyl sulfone and trifluoroalkyl sulfide, which is applied in the field of preparation of trifluoroalkyl sulfone compounds, can solve problems such as the decomposition of hydrogen peroxide, achieve the effects of reducing decomposition, reducing pyrolysis, and improving efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] In one embodiment of the present application, the above-mentioned preparation method includes: continuously feeding the first solution containing trifluoroalkyl sulfide compounds, the aqueous solution containing sodium tungstate dihydrate, and the hydrogen peroxide solution into a continuous reactor respectively .
[0043] In this application, the above-mentioned trifluoroalkyl sulfide compounds and sodium tungstate dihydrate are made into solutions in advance, and then respectively sent into continuous reactors, which is beneficial to the trifluoroalkyl sulfide compounds, tungsten Sodium dihydrate is uniformly dissolved in the solution to form a homogeneous reaction system in the continuous reactor. On the one hand, it helps the above three materials to disperse more uniformly under the same reaction conditions, thereby increasing the probability of collision between the molecules of the reaction materials, thereby improving the efficiency of the reaction; on the other...
Embodiment 1
[0053] Control the temperature at 15-25 °C, and prepare the following materials respectively: Dissolve trifluoromethyl phenylene sulfide (A, 30 g, 0.168 mol) in acetonitrile (270 mL) and stir to clarify, which is designated as material 1; Na 2 WO 4 2H 2 O (5.10 g, 0.015 mol) was dissolved in distilled water (5.4 mL) and stirred to clarify, which was recorded as material 2; 35% H 2 o 2 (81.62 g, 0.84 mol) was recorded as material 3; the retention volume of the continuous coil was 100 mL, and the retention time of the reaction system in the coil was controlled to be 60 min.
[0054] Before feeding, the coil temperature is controlled at 50 °C, and the above-mentioned material 1, material 2 and material 3 are continuously injected into the coil in proportion by using a plunger pump. Among them, the feeding speed of material 1 is 4.06 g / min, the feeding speed of material 2 is 0.18 g / min, and the feeding speed of material 3 is 1.36 g / min. The three materials are fed at the same t...
Embodiment 2
[0056]The temperature was controlled at 15-25 °C, and the following materials were prepared respectively: Dissolve p-chlorotrifluoromethylphenylsulfide (A, 35.72 g, 0.168 mol) in acetonitrile (321.5 mL) and stir to clarify, denoted as material 1; Na 2 WO 4 2H 2 O (5.10 g, 0.015 mol) was dissolved in distilled water (6.43 mL) and stirred to clarify, which was recorded as material 2; 35% H 2 o 2 (81.62 g, 0.84 mol) was recorded as material 3; the retention volume of the continuous coil was 100 mL, and the retention time of the control reaction system in the coil was 60 min.
[0057] Before feeding, the coil temperature is controlled at 50 °C, and the above-mentioned material 1, material 2 and material 3 are continuously injected into the coil in proportion by using a plunger pump. Among them, the feeding speed of material 1 is 4.83 g / min, the feeding speed of material 2 is 0.19 g / min, and the feeding speed of material 3 is 1.36 g / min. The three materials are fed at the same t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com