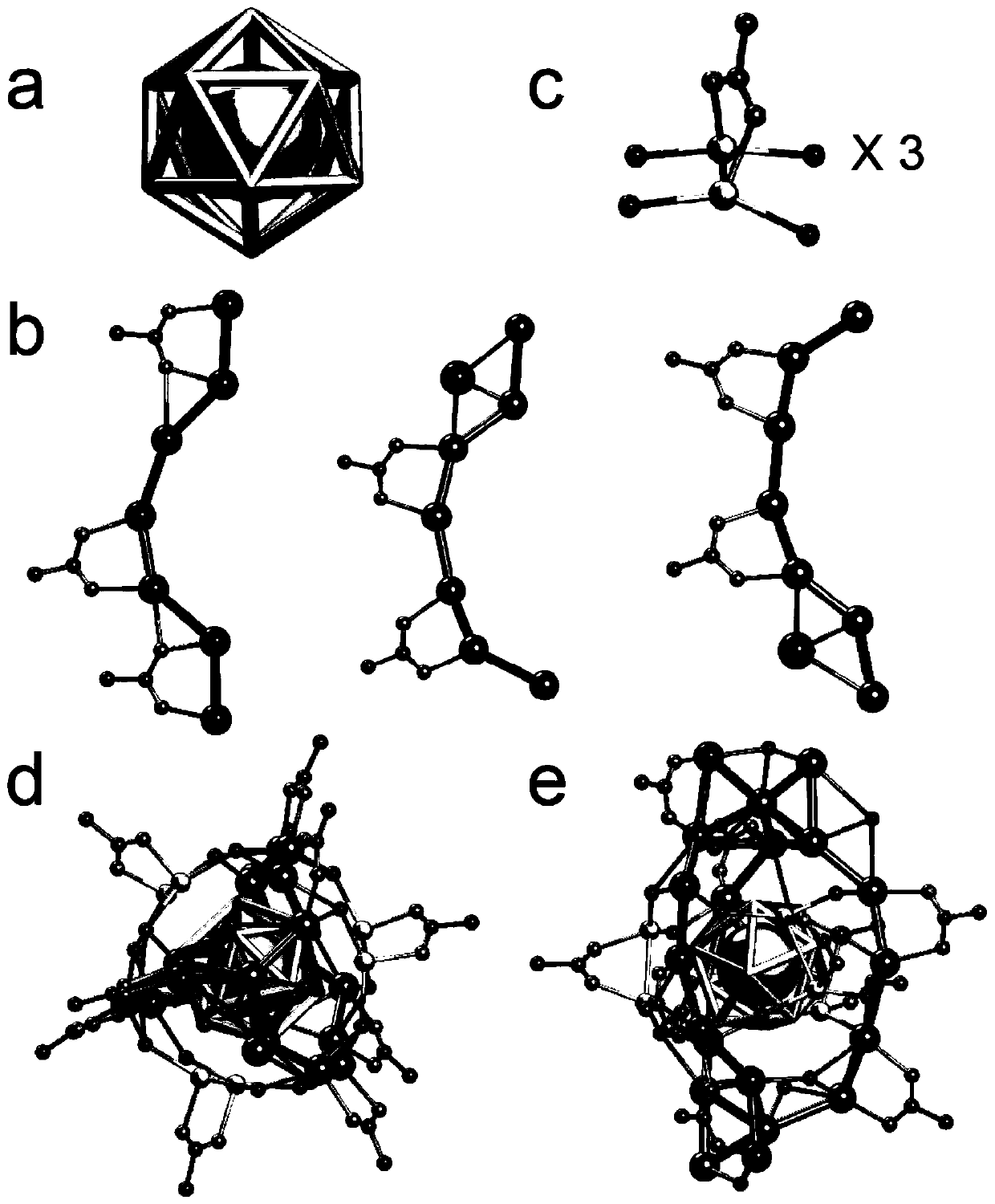
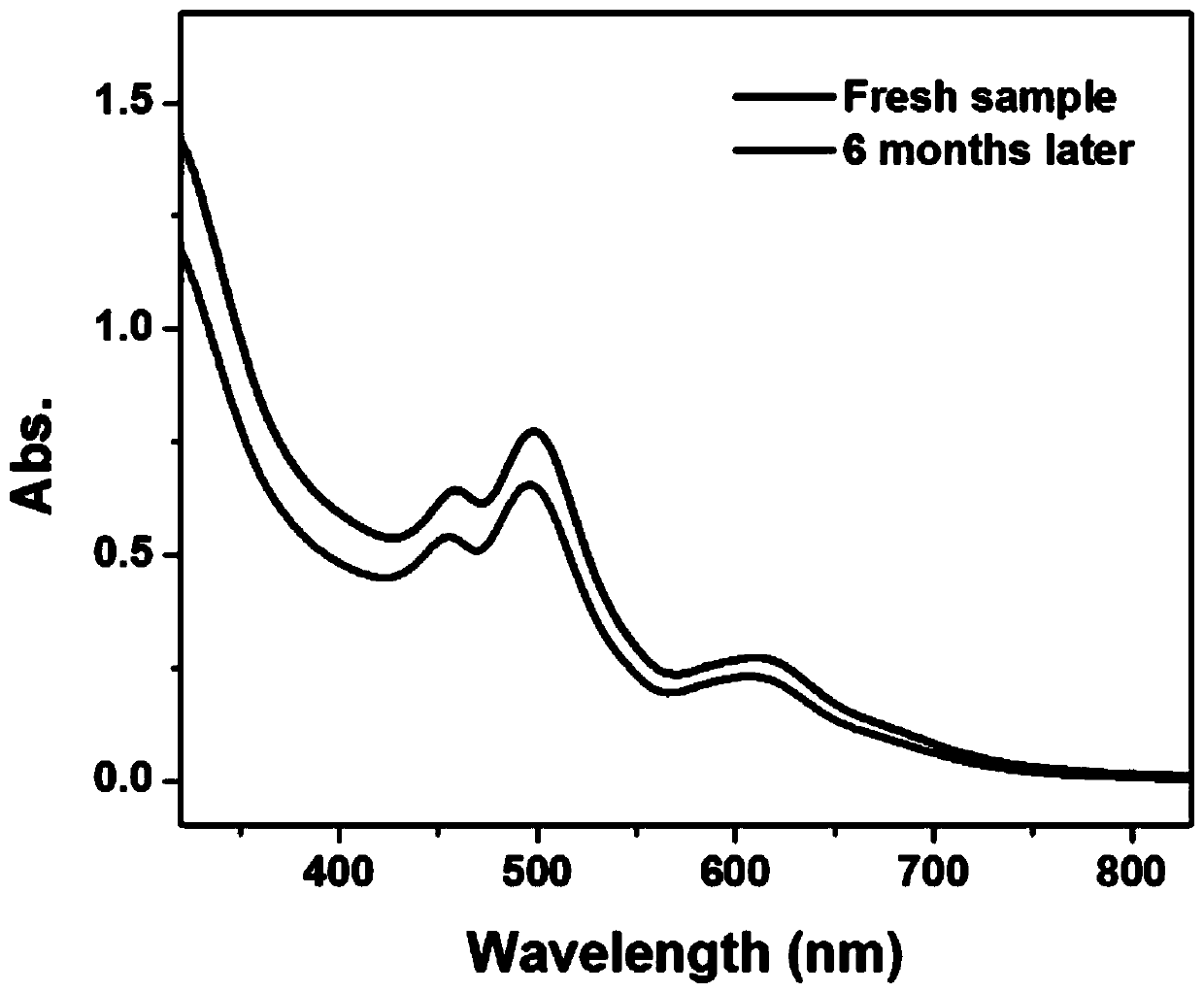
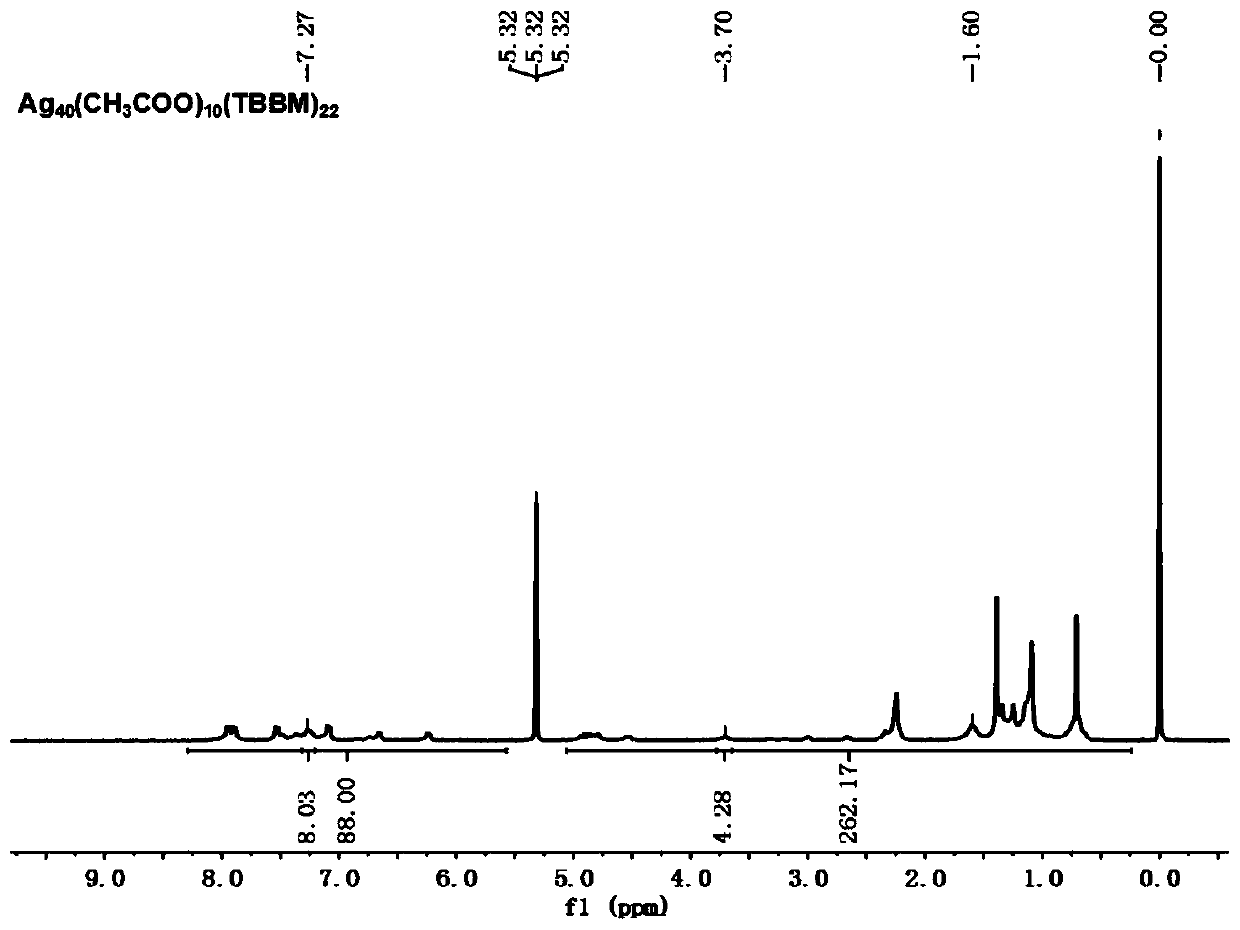
Chiral silver nanocluster and preparation and application thereof
A technology of silver nanoclusters and chirality, which is applied in the fields of silver organic compounds, color/spectral characteristic measurement, organic chemistry, etc. It can solve the problems of complex preparation process, high requirements for instruments, and difficult condition screening, etc., and achieve low raw material cost , Excellent optical performance and good repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0056] The preparation method of the chiral silver nanocluster of the present invention comprises the following steps:
[0057] Add 600mg of silver acetate and 50ml of methanol into a three-necked flask, stir until evenly dispersed, then add 0.4mL of p-tert-butylbenzylmercaptan, react for 30min until the solution is light yellow and turbid, then add 20mL of 1.5g of triacetoxy Sodium borohydride in methanol or tetrahydrofuran solution, react at room temperature for 20 hours; after the reaction, centrifuge first, pour off the supernatant, dissolve the precipitate with 50mL of dichloromethane and centrifuge, and then evaporate the supernatant of dichloromethane with a rotary evaporator The solvent was removed and dried, then dissolved with 50 mL of n-hexane, centrifuged, and the n-hexane supernatant was evaporated with a rotary evaporator to remove the solvent and dried to obtain a crude product; the crude product was recrystallized with dichloromethane: acetonitrile = 1:2 (v / v) t...
Embodiment 1
[0058] Example 1: Ag 40 Concentration detection of R-2-chloropropionic acid in dichloromethane solution
[0059] 1mL of Ag 40 Dichloromethane solution (concentration is 1.16x10 -7 mol / mL) and 1mL of R-2-chloropropionic acid in dichloromethane solution (concentration from 1.16x10 -7 mol / mL to 1.16x10 -6 mol / mL) was uniformly mixed and tested by a circular dichroism spectrometer. The result shows: the concentration of R-2-chloropropionic acid is 1.16x10 -7 mol / mL to 1.16x10 -6 In the range of mol / mL, there is a linear relationship between its circular dichroism spectrum signal and the concentration of R-2-chloropropionic acid, and the linear equation is y=-2.0423x 10 7 C+0.07009, linear correlation coefficient R 2 =0.99976, wherein y is the absorption value at 331 nm on the circular dichroism spectrum, the unit is mdeg, and C is the concentration of R-2-chloropropionic acid, the unit is mol / mL.
Embodiment 2
[0060] Example 2: Ag 40 Measurement of the e.e. value of R-2-chloropropionic acid in dichloromethane solution
[0061] 1mL of Ag 40 Dichloromethane solution (concentration is 1.16x10 -7 mol / mL) and 1mL of R-2-chloropropionic acid in dichloromethane solution (concentration of 1.16x10 -6 mol / mL, e.e. values from 0% to 100%) were uniformly mixed and then tested by a circular dichroism spectrometer. The result shows: the e.e. value of R-2-chloropropionic acid ranges from 0% to 100%, and its circular dichroism spectrum signal is linearly related to the e.e. value of R-2-chloropropionic acid, and the linear equation is y=- 0.24382C+0.46794, linear correlation coefficient R 2 =0.99949, wherein y is the absorption value at 331 nm on the circular dichroism spectrum, the unit is mdeg, and C is the e.e. value of R-2-chloropropionic acid, the unit is %.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com