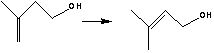Preparation method of allylic alcohol
A technology of isopentenol and butene, applied in the direction of isomerization preparation, chemical instruments and methods, organic compound/hydride/coordination complex catalyst, etc., can solve difficult guarantee, difficult separation, affecting yield, etc. problems, to achieve the effect of high raw material conversion rate and product selectivity, low reaction temperature, and many application times
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Weigh 0.165g of bisphosphine ligand 1,3-bis(diphenylphosphine)propane (DPPP), 0.089g of transition metal compound such as palladium acetate, add them into a three-necked flask, add 100ml of acetone, replace with nitrogen for 3 times, at 28°C Stir for 25 minutes, then stop the reaction, and spin dry at 50° C. to obtain 0.15 g of the complex catalyst with the chelate structure.
[0030] Add 150 grams of 3-methyl-3-buten-1-ol and 0.15 g of the catalyst prepared by the above method into a 500 m autoclave, replace the nitrogen three times, keep the hydrogen pressure at 0.7 Mpa, control the temperature at 55 ° C, and rotate at 200 rpm After reacting for 60 minutes, stop the reaction, ventilate and filter to obtain 150 g of prenol, with a selectivity of 95.21% and a conversion rate of 79.18%.
Embodiment 2
[0032] Weigh 0.165g of bisphosphine ligand 1,3-bis(diphenylphosphine)propane (DPPP) and 0.098g of transition metal compound such as palladium acetate into a three-neck flask, add 150ml of acetone, replace with nitrogen for 3 times, at 25°C Stir for 30 minutes, then stop the reaction, and spin dry at 50° C. to obtain 0.16 g of the complex catalyst with the chelate structure.
[0033] Add 150 grams of 3-methyl-3-buten-1-ol and 0.075 g of the catalyst prepared by the above method into a 500m autoclave, replace with nitrogen three times, keep the pressure of hydrogen at 1.0Mpa, control the temperature at 50°C, and rotate at 50 rpm After reacting for 20 minutes, the reaction was stopped, and the gas was ventilated and filtered to obtain 169.1 g of prenol, with a selectivity of 95.34% and a conversion rate of 89.26%.
Embodiment 3
[0035]Weigh 0.165g of bisphosphine ligand 1,3-bis(diphenylphosphine)propane (DPPP), 0.108g of transition metal compound such as palladium acetate (molar ratio 1:1.2), add them into a three-necked flask, add 150ml of acetone, Replaced with nitrogen three times, stirred at 23°C for 35 minutes, then stopped the reaction, and spin-dried in vacuum at 50°C to obtain 0.14 g of the complex catalyst with the chelate structure.
[0036] Add 150g of 3-methyl-3-buten-1-ol and 0.105g of the catalyst prepared by the above method into a 500m autoclave, replace with nitrogen 3 times, keep the pressure of hydrogen at 1.2Mpa, control the temperature at 70°C, and react at 400 rpm for 90 minutes, the reaction was stopped, and the gas was vented and filtered to obtain 174.8 g of prenol, with a selectivity of 95.97% and a conversion rate of 92.35%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com