Synthesis method of alpha-acetyl-gamma-butyrolactone
A synthetic method, the technology of butyrolactone, applied in the direction of organic chemistry, can solve the problems of large environmental pollution, no investment, difficult implementation, etc., and achieve the effects of eliminating pollution sources, preventing instability, and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The first reaction kettle is a storage tank, and the storage tank is replaced with nitrogen. In a fully dry storage tank (20L) with a thermometer and a heat-conducting oil jacket, put 3.4kg of sodium metal into it under a nitrogen atmosphere, turn on the heat-conducting oil for heating, and when the temperature reaches 130°C, keep it warm for 2 hours to make the metal All the sodium is melted to obtain liquid metallic sodium in a molten state.
[0034] The second reactor is a condensation reactor. Add 16kg of γ-butyrolactone and 49.5kg of ethyl acetate into a fully dried condensation reaction kettle (100L) equipped with an electric stirrer, a condenser, and a thermometer, stir, and then slowly heat up to raise the temperature. When the system is refluxed, open the The dripping valve of the storage tank, according to the dropping rate of 2.5kg / h, drips the liquid metal sodium in the molten state into the condensation reactor. As the dripping proceeds, the temperature in...
Embodiment 2-32
[0037] Examples 2-32: α-acetyl-γ-butyrolactone was synthesized according to the method in Example 1 using the raw materials and parameters of each example in Table 1.
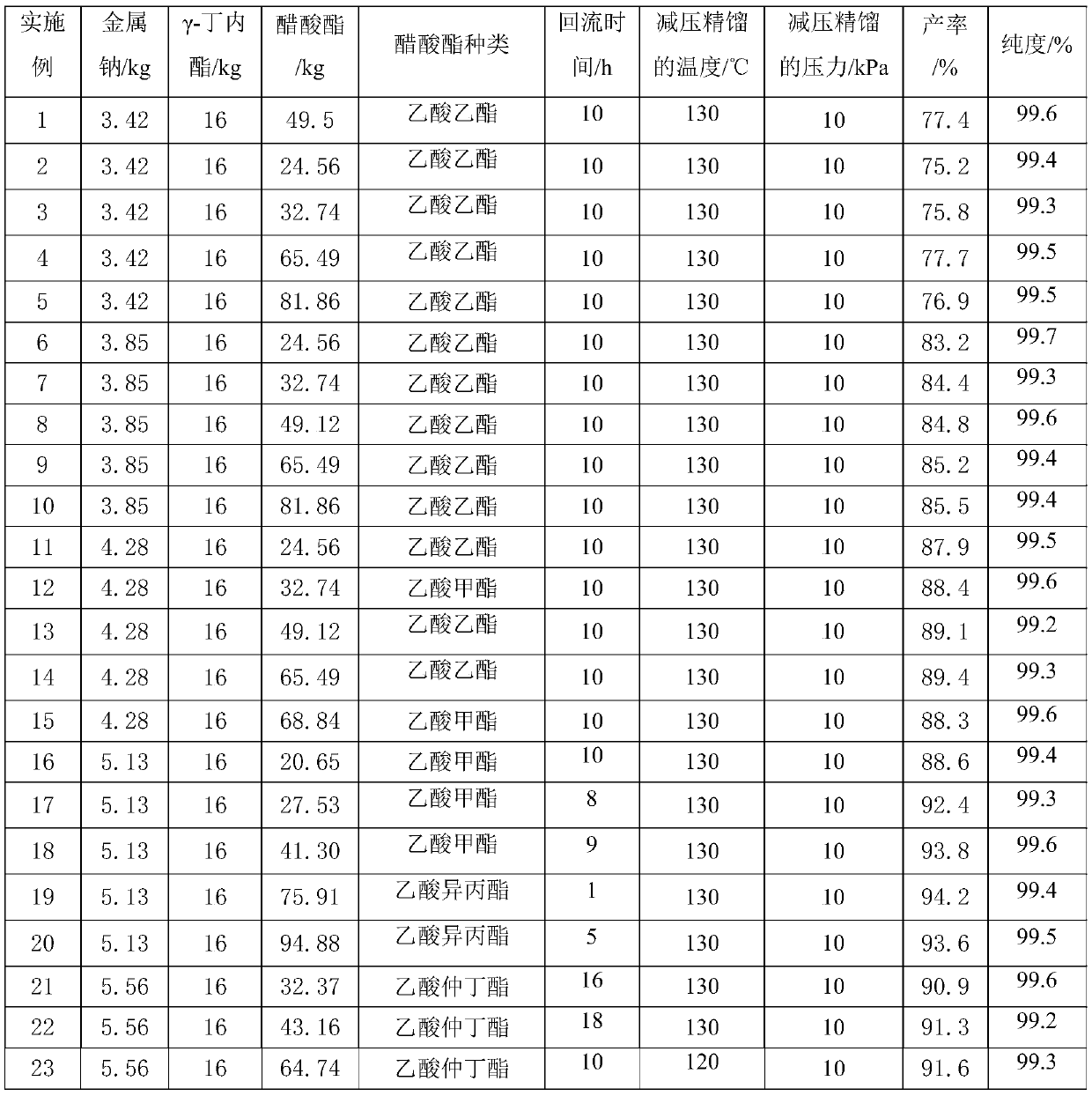
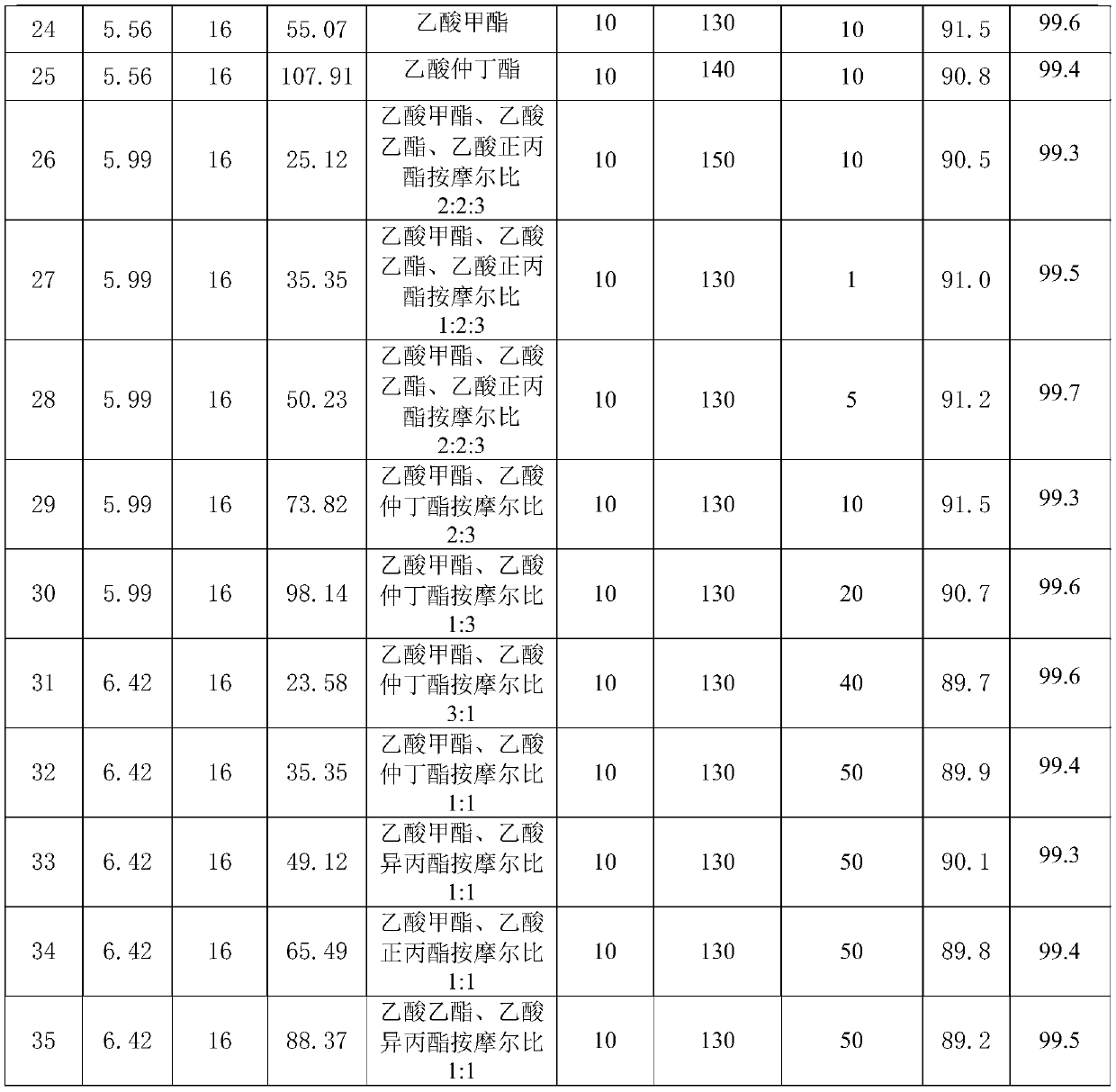
[0038] Raw material parameter and result in the embodiment 1-32 of table 1
[0039]
[0040]
[0041] In summary, the synthesis method of α-acetyl-γ-butyrolactone of the present invention does not need to add benzene as a reaction solvent or extractant, and is not only safe but also has high purity and high yield, with a purity greater than 99.0% and a yield greater than 87.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com