Ultrafine bimetallic IrRu nanowire catalyst and preparation and application thereof
A nano-catalyst, bimetallic nano-technology, applied in metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, chemical instruments and methods, etc., can solve the problem of slow exchange current density, lack of high activity and high Stability, hindering AAEMFC anode to reduce precious metal loading and other issues, to achieve high hydrogen oxidation catalytic activity, good hydrogen oxidation catalytic activity and stability, good effect of stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] preparation:
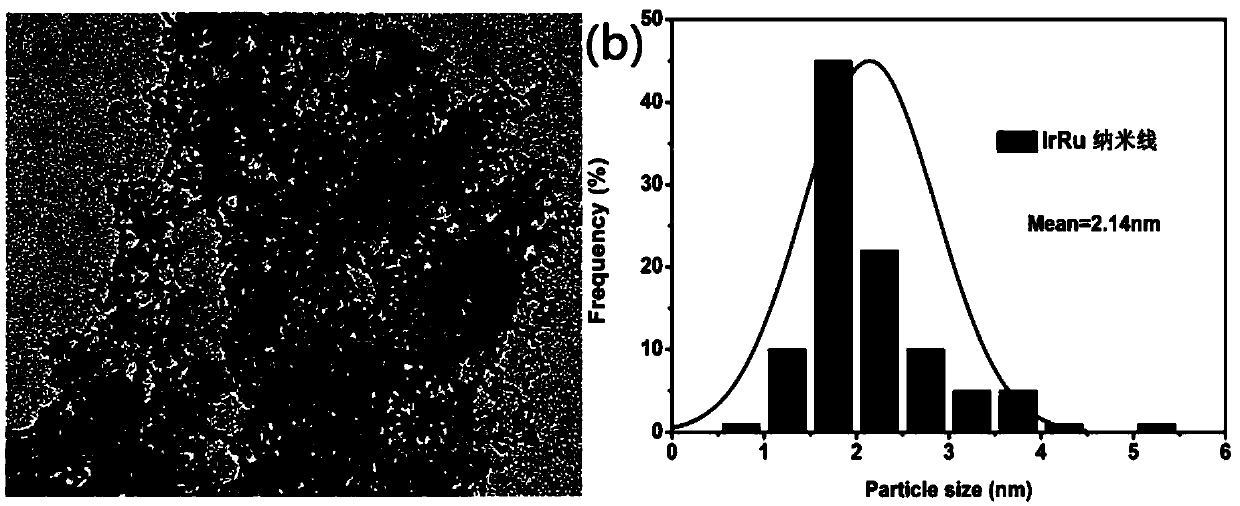
[0026] (1) Preparation of IrRu nanowires: Take 1g of cetyltrimethylammonium bromide (CTAB), 20mL of n-hexane, and 10mL of n-hexanol in a 50mL three-neck flask in an oil bath at 40°C, stir for 30min, and then Add 0.05mM H 2 IrCl 6 , 0.05mM RuCl 3 aqueous solution, continue to stir for more than 3 hours, then add 5 mL of 2M NaBH 4 Fresh aqueous solution was reacted at 40°C for 12 hours to obtain a reaction slurry of IrRu nanowires, which was washed by centrifugation and vacuum dried to prepare IrRu nanowires with an Ir:Ru atomic ratio of 1:1.
[0027] (2) Preparation of IrRu NWs / C catalyst: Take the reaction slurry of IrRu nanowires prepared in (1), add 50 mg of XC-72, and stir for 12 hours. The IrRuNWs / C nanowire catalyst with a mass fraction of metal of 20% was prepared by centrifugal washing and vacuum drying.
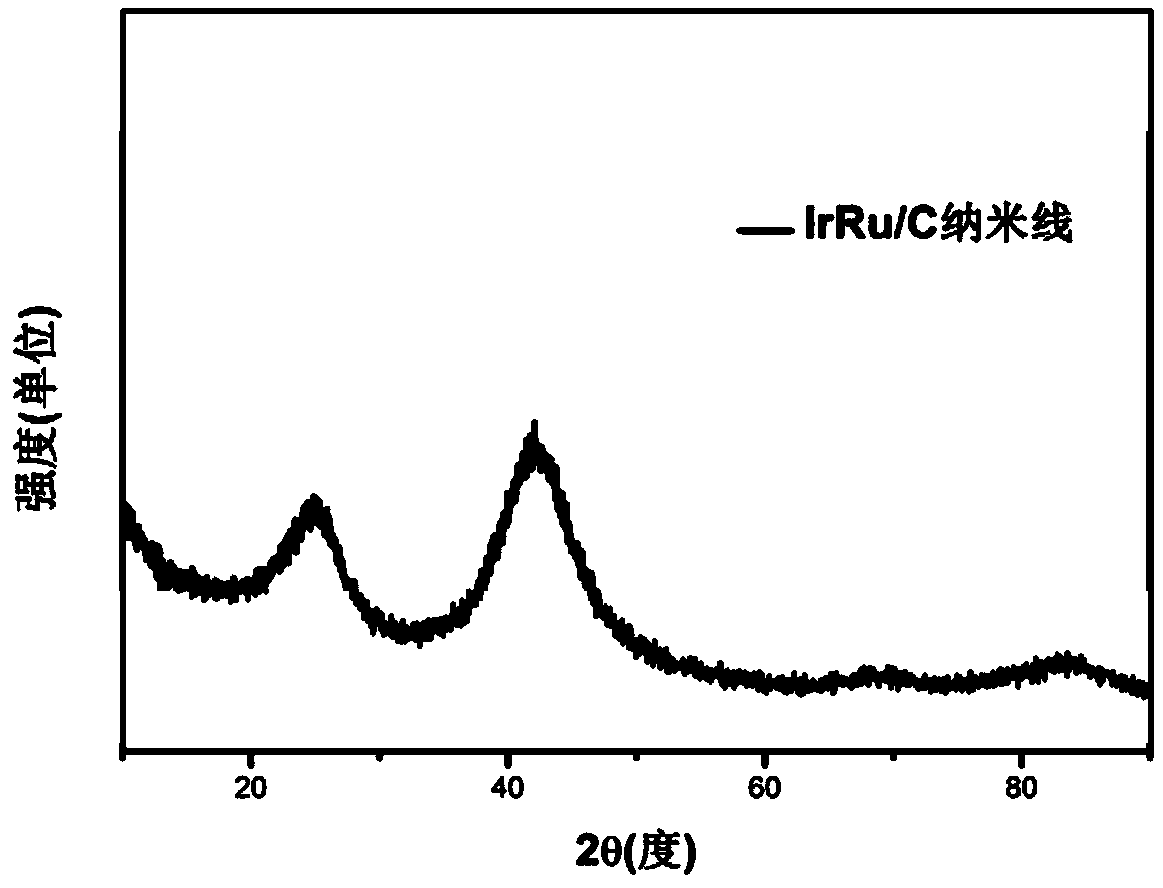
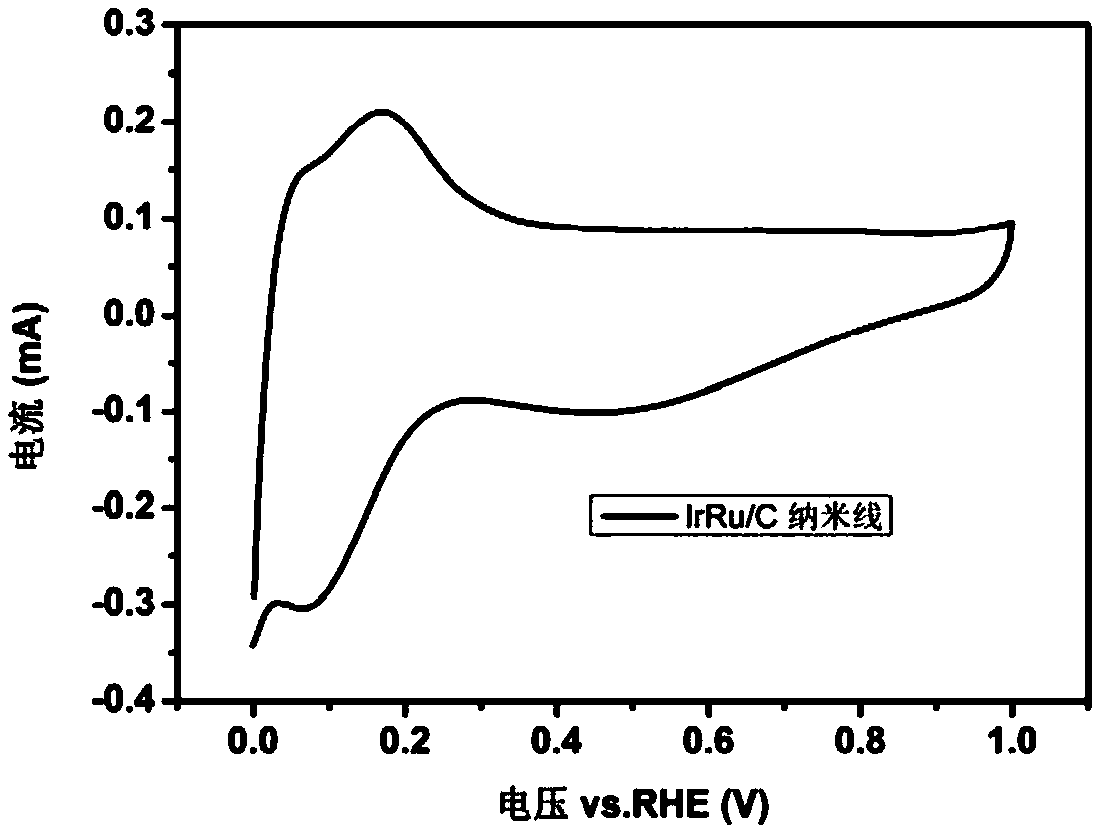
[0028] Get the prepared IrRu NWs / C catalyst of step (2) among the appropriate example 1 and carry out powder XRD test, the result is as fol...
Embodiment 2
[0038] preparation:
[0039] (1) Preparation of IrRu nanowires: Take 1g of cetyltrimethylammonium bromide (CTAB), 20mL of n-hexane, and 10mL of n-hexanol in a 50mL three-neck flask in an oil bath at 40°C, stir for 30min, and then Add 0.05mM H 2 IrCl 6 , 0.05mM RuCl 3 aqueous solution, continue to stir for more than 3 hours, then add 5 mL of 2M NaBH 4 Fresh aqueous solution was reacted at 40°C for 12 hours to obtain a reaction slurry of IrRu nanowires, which was washed by centrifugation and vacuum dried to prepare IrRu nanowires with an Ir:Ru atomic ratio of 1:1.
[0040] (2) Preparation of IrRu NWs / C catalyst: Take the reaction slurry of IrRu nanowires prepared in (1), add 200 mg of XC-72, and stir for 12 hours. The IrRu NWs / C nanowire catalyst with a metal mass fraction of 5% and an Ir:Ru atomic ratio of 1:1 was prepared by centrifugal washing and vacuum drying.
Embodiment 3
[0042] preparation:
[0043] (1) Preparation of IrRu nanowires: Take 1g of cetyltrimethylammonium bromide (CTAB), 20mL of n-hexane, and 10mL of n-hexanol in a 50mL three-neck flask in an oil bath at 40°C, stir for 30min, and then Add 0.05mM H 2 IrCl 6 , 0.05mM RuCl 3 aqueous solution, continue to stir for more than 3 hours, then add 5 mL of 2M NaBH 4 Fresh aqueous solution was reacted at 40°C for 12 hours to obtain a reaction slurry of IrRu nanowires, which was washed by centrifugation and vacuum dried to prepare IrRu nanowires with an Ir:Ru atomic ratio of 1:1.
[0044] (2) Preparation of IrRu NWs / C catalyst: Take the reaction slurry of IrRu nanowires prepared in (1), add 5.5 mg of XC-72, and stir for 12 hours. The IrRu NWs / C nanowire catalyst with a metal mass fraction of 70% and an Ir:Ru atomic ratio of 1:1 was prepared by centrifugal washing and vacuum drying.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com