Catalyst for hydrogenolysis and preparation method thereof
A catalyst and oxide technology, used in catalysts, hydrogenation to hydrocarbons, molecular sieve catalysts, etc., can solve the problems of low reaction conversion rate of copper-based catalysts, high reaction conditions, high reaction temperature and pressure, etc., and achieve high conversion rate. and the effect of target product selectivity, high reactivity, high conversion and selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
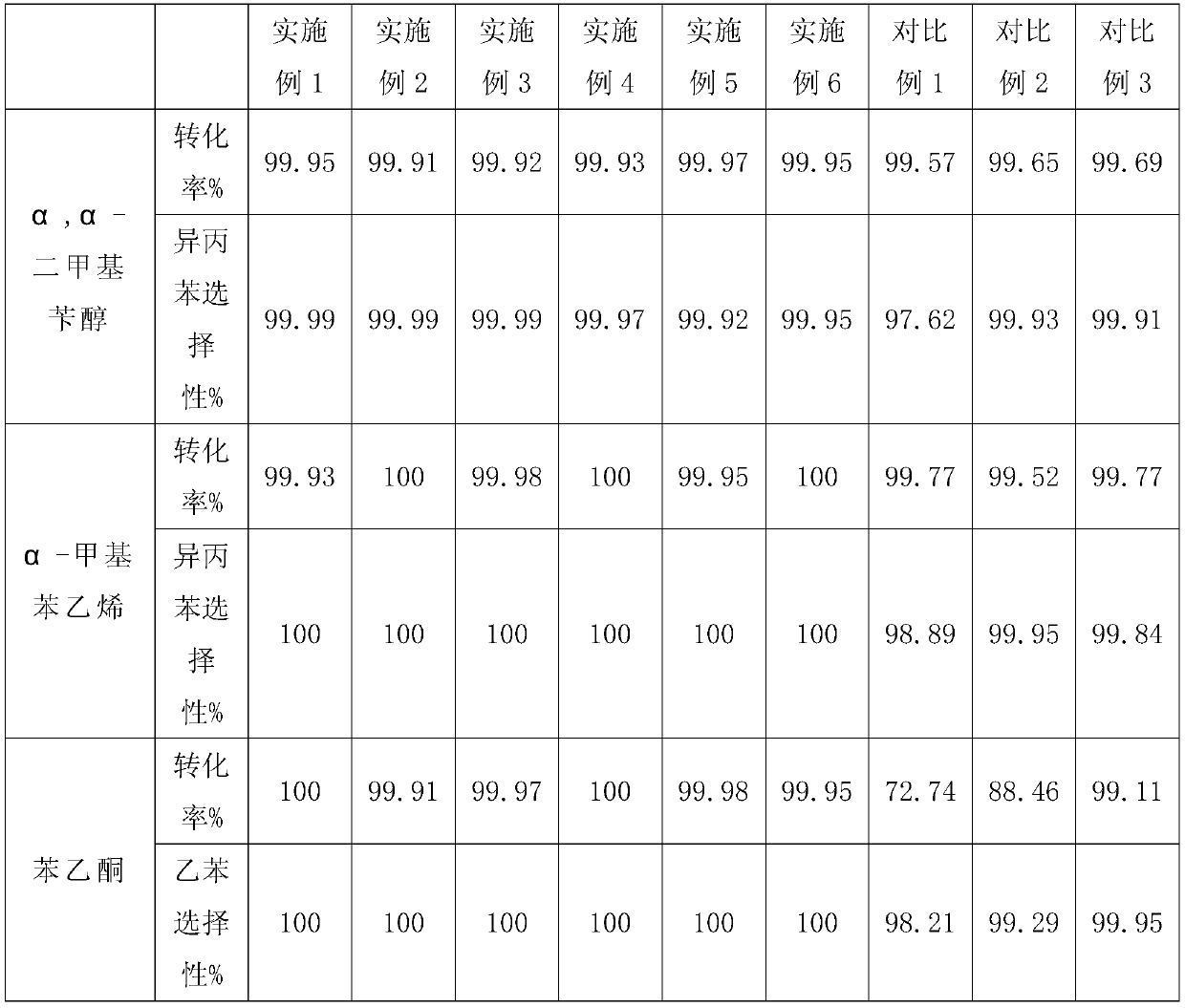
Embodiment 1
[0035]Catalyst #1 contains 80.0% copper, 2.0% nickel, 0.01% palladium, 0.5% chromium oxide, 1.5% zinc oxide, and 15.99% aluminum oxide in mass percent.
[0036] Prepare a first metal salt solution with a total concentration of 0.5 mol / L, and a second metal salt solution with a total concentration of 0.5 mol / L, the second metal salt solution is a mixed salt solution of chromium nitrate and zinc nitrate.
[0037] In a reaction vessel with a stirring paddle, put 1.5L of deionized water into it, and after raising the temperature to 80°C, add 0.5mol / L sodium carbonate solution and the first metal salt solution dropwise at the same time while stirring, and the sodium carbonate solution and the first metal salt solution The mass ratio of saline solution is 2:1. After the dropwise addition, the reaction was continued at 80° C. for 2 h, the pH was adjusted to 7.5-8.0, and the required amount of the second metal salt solution and alumina were added. After fully reacting, it is filtered...
Embodiment 2
[0039] Catalyst 2# contains 40.0% copper, 8.0% nickel, 0.05% palladium, 1.0% magnesium oxide, 0.5% barium oxide, 0.5% lead oxide, 1.5% chromium oxide and 48.45% silicon oxide in mass percent.
[0040] Prepare the first metal salt solution with a total concentration of 0.6mol / L, and the second metal salt solution with a total concentration of 0.6mol / L, the second metal salt solution is a mixture of magnesium hydrochloride, barium hydrochloride, lead hydrochloride and chromium hydrochloride saline solution.
[0041] Put 1.0L of deionized water into a reaction vessel with a stirring paddle, and after heating up to 85°C, add 0.5 mol / L sodium carbonate solution and the first metal salt solution dropwise under stirring at the same time, the sodium carbonate solution and the first metal salt solution The mass ratio of saline solution is 3:1. After the dropwise addition, the reaction was continued at 85° C. for 1.5 h, the pH was adjusted to 8.5-9.0, and the required amount of the sec...
Embodiment 3
[0043] Catalyst 3# contains 60.0% copper, 3.0% nickel, 0.01% palladium, 1.5% zinc oxide, 1.0% lead oxide, 1.5% chromium oxide and 32.99% molecular sieve in mass percentage.
[0044] Prepare the first metal salt solution with a total concentration of 0.2mol / L, and the second metal salt solution with a total concentration of 0.2mol / L, the second metal salt solution is a mixed salt solution of zinc sulfate, lead sulfate and chromium sulfate.
[0045] In a reaction vessel with a stirring paddle, put 1.5L of deionized water into it, and after raising the temperature to 90°C, add 0.3mol / L sodium carbonate solution and the first metal salt solution dropwise under stirring at the same time, and the sodium carbonate solution and the first metal salt solution The mass ratio of saline solution is 2:1. After the dropwise addition, the reaction was continued at 90° C. for 0.5 h, the pH was adjusted to 7.5-8.0, and the required amount of the second metal salt solution and molecular sieves w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com