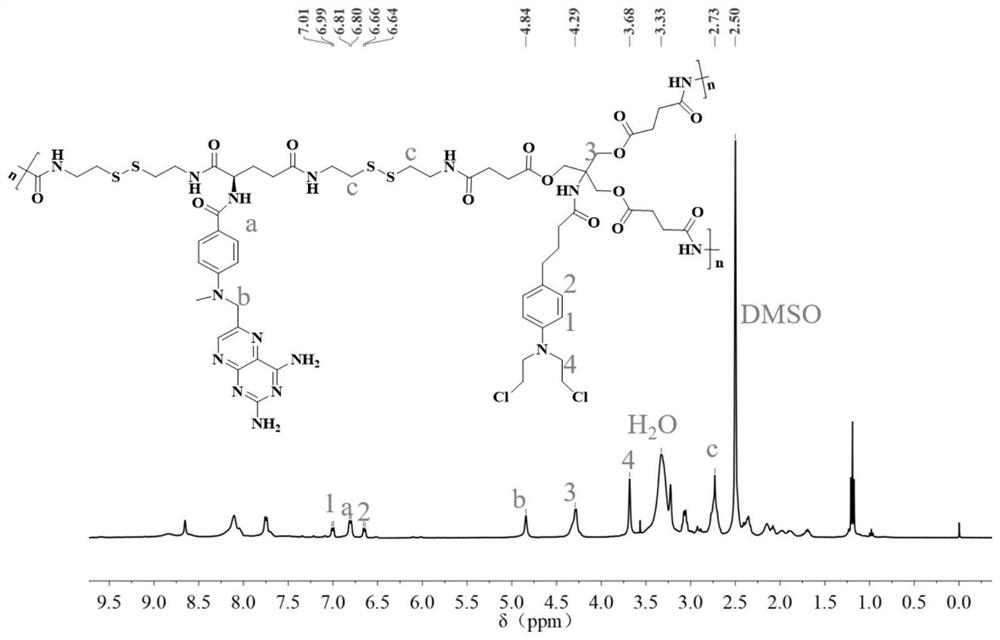
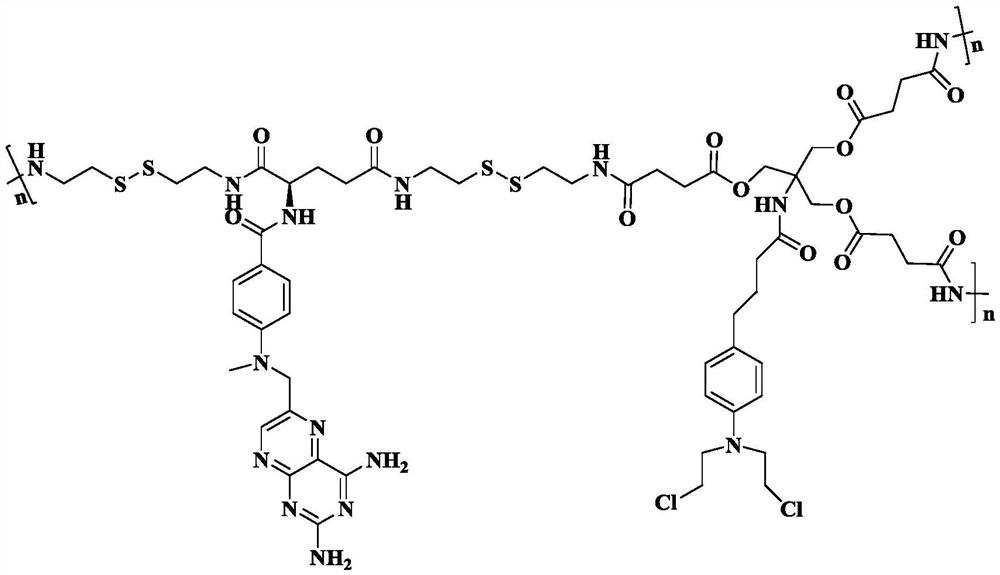
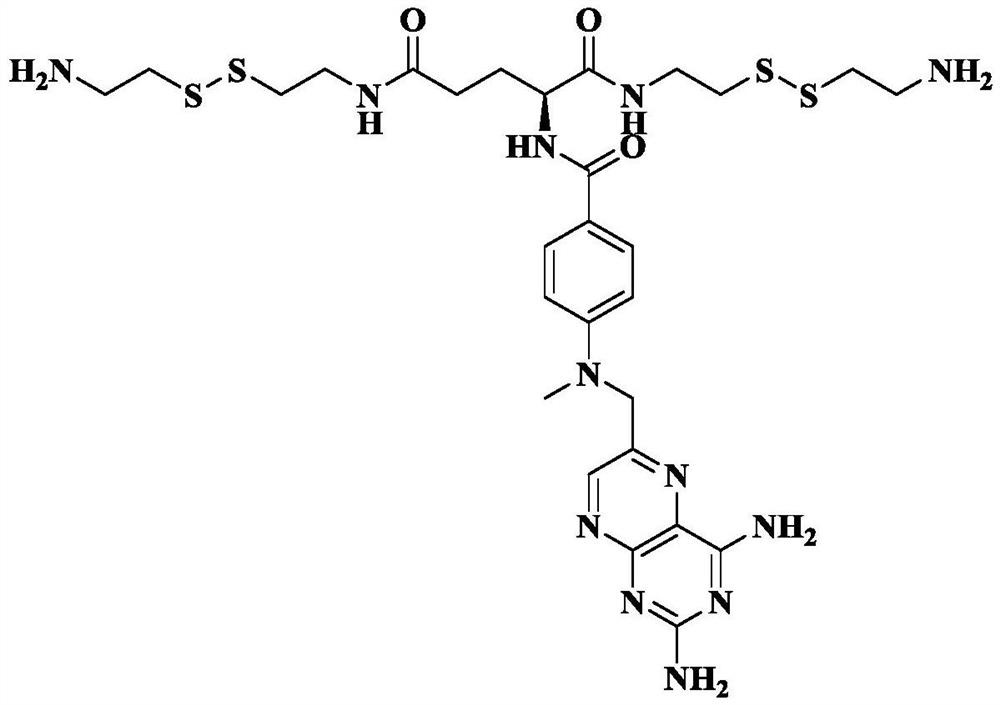
A kind of carrier-free hyperbranched macromolecular polymer and its preparation method
A macromolecular polymer and carrier-free technology, which is applied in the direction of medical preparations with non-active ingredients, medical preparations containing active ingredients, drug combinations, etc., can solve the problem of cumbersome synthetic routes, unsimple synthetic methods, and system drug loading rate. Not high problem, to solve the effect of low drug loading
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Weigh 250mg of cystamine dihydrochloride and place it in a dry 100ml single-necked flask, add 5ml of methanol and 350mg of triethylamine, and stir for 30min under ice-bath conditions. Weigh 88 mg of di-tert-butyl dicarbonate into a beaker and dissolve it with 10 ml of methanol, and add it dropwise into a single-necked flask with a constant pressure dropping funnel. After reacting for 5 hours, add 10 ml of deionized water to terminate the reaction. The reaction solvent was removed by rotary evaporation under reduced pressure, then 30 ml of saturated sodium chloride solution was added, extracted three times with 50 ml of dichloromethane, and the organic phase was dried over anhydrous sodium sulfate. The resulting solid was directly applied to a silica gel column for separation. The solvent was evaporated to dryness with a rotary evaporator, and dried in a vacuum oven for 2 days to obtain 123 mg of cystamine with a single-tert-butoxycarbonyl-protected amino group as a pale...
Embodiment 2
[0050] Weigh 400mg of cystamine dihydrochloride and place it in a dry 100ml single-necked flask, add 8ml of methanol and 560mg of triethylamine, and stir for 30min under ice-bath conditions. Weigh 140 mg of di-tert-butyl dicarbonate into a beaker and dissolve it with 12 ml of methanol, and add it dropwise into a single-necked flask with a constant pressure dropping funnel. After reacting for 5 hours, add 10 ml of deionized water to terminate the reaction. The reaction solvent was removed by rotary evaporation under reduced pressure, then 30 ml of saturated sodium chloride solution was added, extracted three times with 50 ml of dichloromethane, and the organic phase was dried over anhydrous sodium sulfate. The resulting solid was directly applied to a silica gel column for separation. The solvent was evaporated to dryness with a rotary evaporator, and dried in a vacuum oven for 2 days to obtain 196 mg of cystamine with a single-tert-butoxycarbonyl-protected amino group as a pal...
Embodiment 3
[0057] Weigh 500mg of cystamine dihydrochloride and place it in a dry 100ml single-necked flask, add 10ml of methanol and 700mg of triethylamine, and stir for 30min under ice-bath conditions. Weigh 175 mg of di-tert-butyl dicarbonate into a beaker and dissolve it with 15 ml of methanol, and add it dropwise into a single-necked flask with a constant pressure dropping funnel. After reacting for 5 hours, add 10 ml of deionized water to terminate the reaction. The reaction solvent was removed by rotary evaporation under reduced pressure, then 30 ml of saturated sodium chloride solution was added, extracted three times with 50 ml of dichloromethane, and the organic phase was dried over anhydrous sodium sulfate. The obtained solid was directly applied to a silica gel column for separation, the solvent was evaporated to dryness with a rotary evaporator, and dried in a vacuum oven for 2 days to obtain 257 mg of cystamine with a single-terminal tert-butoxycarbonyl-protected amino group ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com