Polymerizable lignin-based macromolecular photoinitiator, preparation method and applications thereof
A photoinitiator, lignin-based technology, applied in the field of photocuring, can solve the problems of complex synthesis, few functional groups, high risk or high cost, and achieve improved comprehensive performance, improved macroscopic performance, and high photoinitiated activity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] This embodiment provides an ester-soluble polymerizable lignin-based macromolecular photoinitiator L-AC-2959, which is prepared according to the following steps:
[0065] 1) Dissolve 20 g of alkaline lignin in 1 mol / L aqueous sodium hydroxide solution to make the lignin content 25% (w / w), and then add 21.3 mL of triethylamine to the above solution. The above solution was placed in an ice-water mixed bath and the temperature was controlled at 0-5°C. Under vigorous stirring, a mixed solution of 9.05 g of acryloyl chloride and dichloromethane (18 mL) was slowly added dropwise. After the dropwise addition was completed, the reaction was continued at this temperature for a period of time. The pH of the obtained reaction solution was adjusted to 7 with hydrochloric acid, and a large amount of brown-black solids were precipitated. The precipitates were obtained by filtration and dried to obtain a crude product (L-AC).
[0066]
[0067] Wherein, n1 and n4 are natural number...
Embodiment 2
[0074] Similar to step 3) of Example 1, add 8.0 g of the product obtained in step 2) of Example 1 and 5.0 g of the product obtained in step 1) of Example 1 and 300 mL of dimethyl sulfoxide solvent in a 500 mL there-necked flask, and then in the above-mentioned Add 0.1 mol / L sodium hydroxide dropwise to the solution to adjust the pH between 8 and 9, heat to 80°C and react under this condition for 4 hours. After the reaction, cool down naturally, adjust the pH of the reaction solution to 7 with hydrochloric acid, then wash the reaction solution with a small amount of water, filter, retain the brown aqueous solution, and then use a dialysis membrane with a molecular weight cut-off of 5kDa for 7 days to remove residual small molecules. Photoinitiators, solvents, salts formed, and other impurities. The resulting brown aqueous solution in the dialysis membrane was poured into a large watch glass and dried under vacuum at 30°C for 7 days to obtain the pure final product L-AC-2959.
...
Embodiment 3
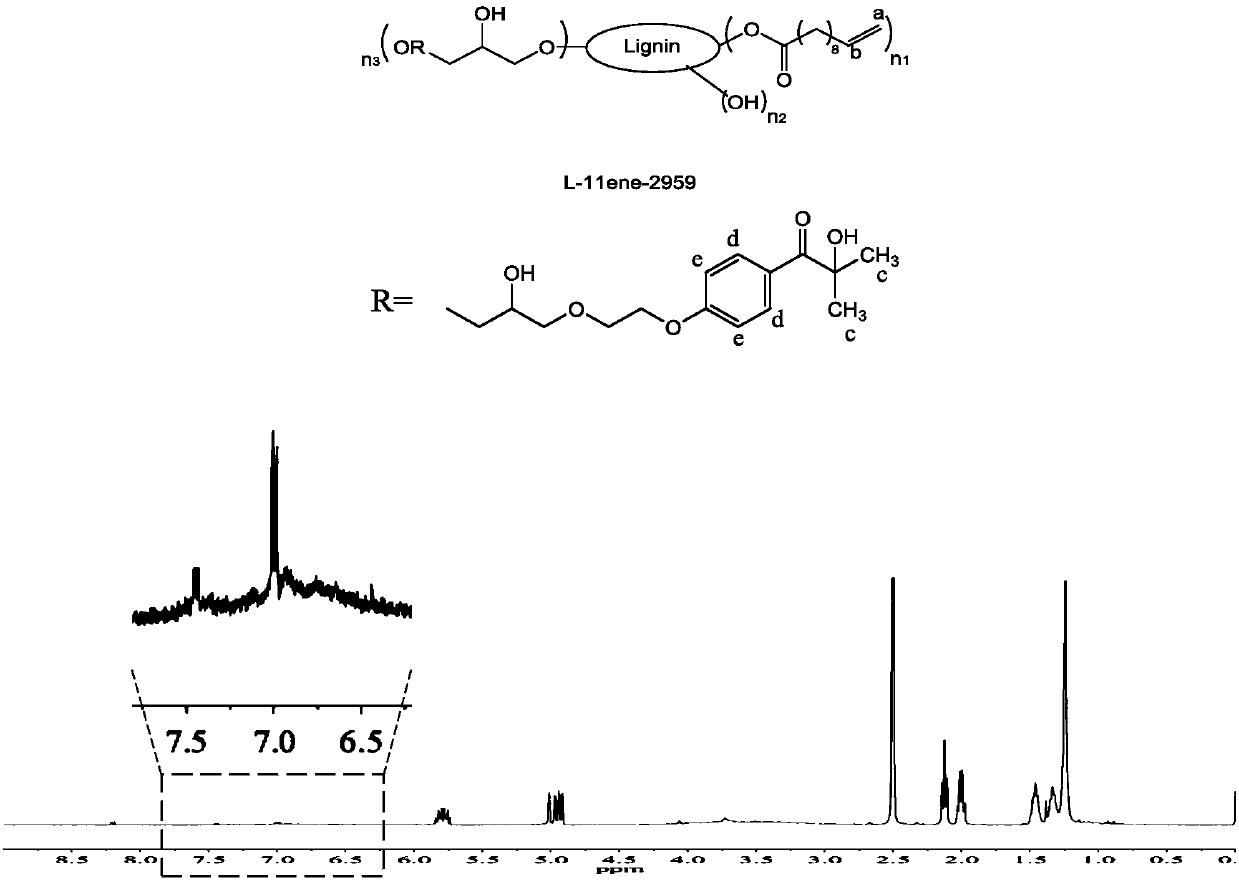
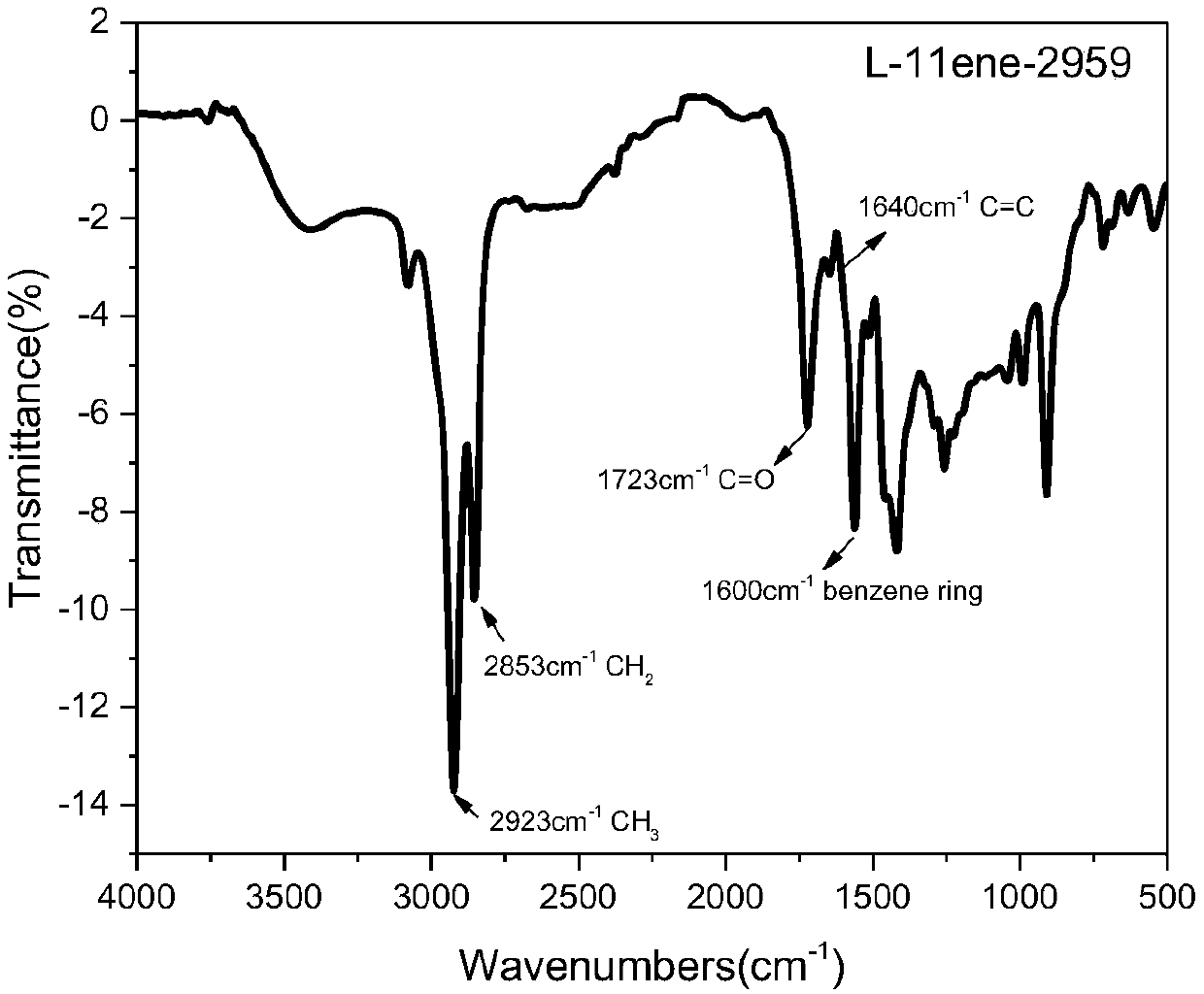
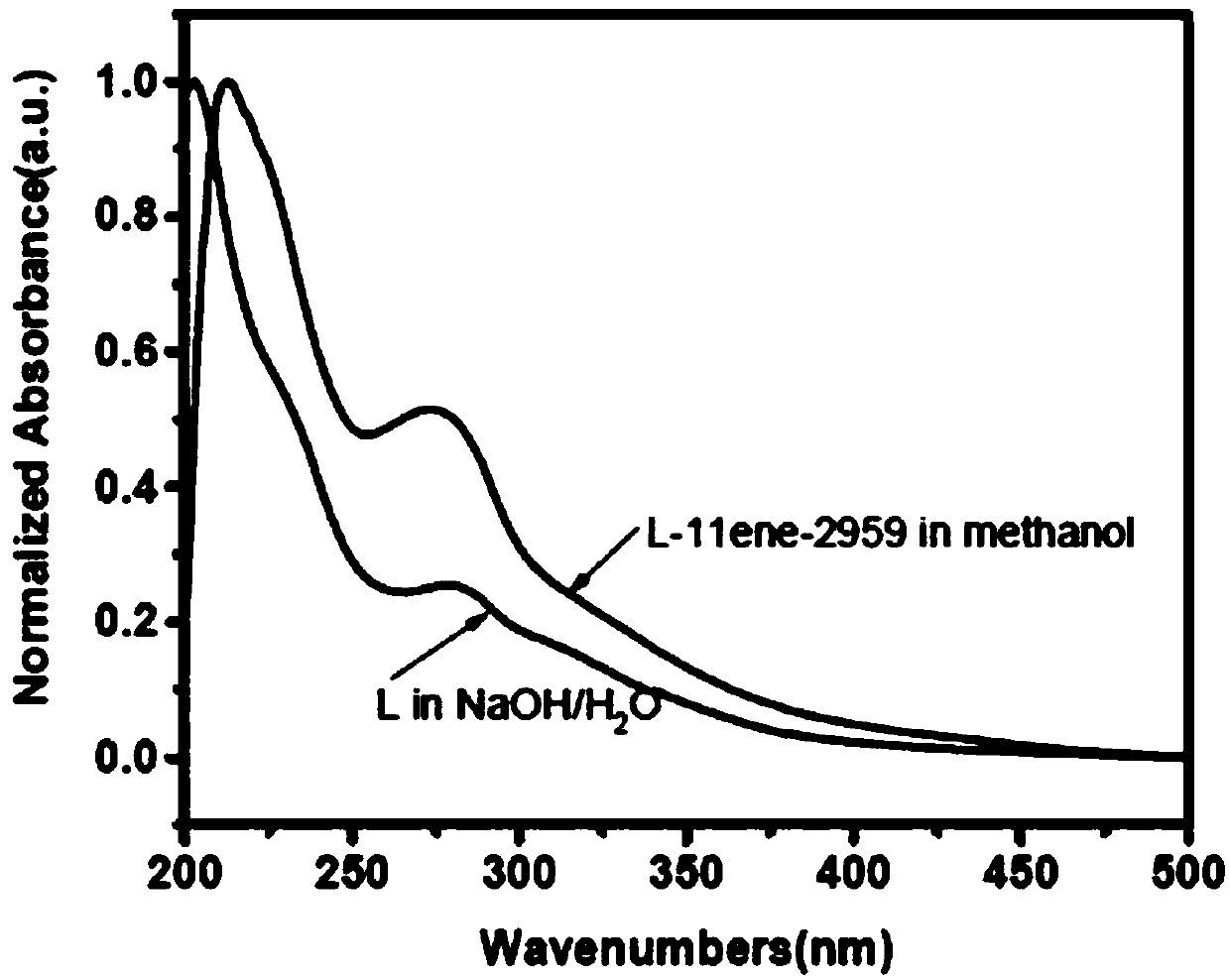
[0077] This example provides an ester-soluble polymerizable lignin-based macromolecular photoinitiator L-11ene-2959, which is prepared according to the following steps:
[0078] 1) Dissolve 20 g of alkaline lignin in 1 mol / L aqueous sodium hydroxide solution to make the lignin content 25% (w / w), and then add 21.3 mL of triethylamine to the above solution. The above solution was placed in an ice-water mixed bath and the temperature was controlled at 0-5°C. Under vigorous stirring, a mixed solution of 20.2 g of undecylenoyl chloride and dichloromethane (40 mL) was slowly added dropwise. After the dropwise addition was completed, the reaction was continued at this temperature for a period of time. The pH of the obtained reaction solution was adjusted to 7 with hydrochloric acid, and then unreacted unreacted acid chloride and the by-product undecylenic acid were extracted with a large amount of petroleum ether, and finally the crude product (L-11ene) was obtained by filtration and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com