Aspergillus for producing monacolin J, and construction method and application thereof
A technology for producing monacolin and constructing a method, which is applied in the field of biopharmaceuticals, can solve the problems of large differences in lovastatin production, easy degradation, and accumulation, and achieve the effects of enhancing fermentation stability, simplifying the production process, and reducing environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Embodiment 1, Aspergillus genetic transformation
[0075] 1. Protoplast preparation
[0076] The spores of Aspergillus HZ01 were inoculated into 50mL IPM liquid medium, so that the spore concentration was about 10 7 cells / mL, cultured at 200rmp, 32°C for 12-18h. Collect the growing mycelium by filtering with a sterile single layer of 500-mesh nylon cloth, and filter with sterile 0.6M MgSO 4 The solution was rinsed three times, pressed dry and placed in a sterile 50ml Erlenmeyer flask, adding an appropriate amount of enzymatic solution according to the weight of mycelium (add 10ml of enzymatic solution per 1g of mycelium), and treated at 30°C and 60rpm for 1-3h. Filter the mixture after the above enzymolysis with 8 layers of lens-cleaning paper, and collect the filtrate. Collect protoplasts by centrifugation at 4°C and 4000rpm, wash once with pre-cooled 1.0M sorbitol solution, and then wash with pre-cooled STC (STC composition: 1.0M sorbitol, 50mM Tris-HCl (pH 8.0), 5...
Embodiment 2
[0079] Example 2, complete knockout of the lovF gene
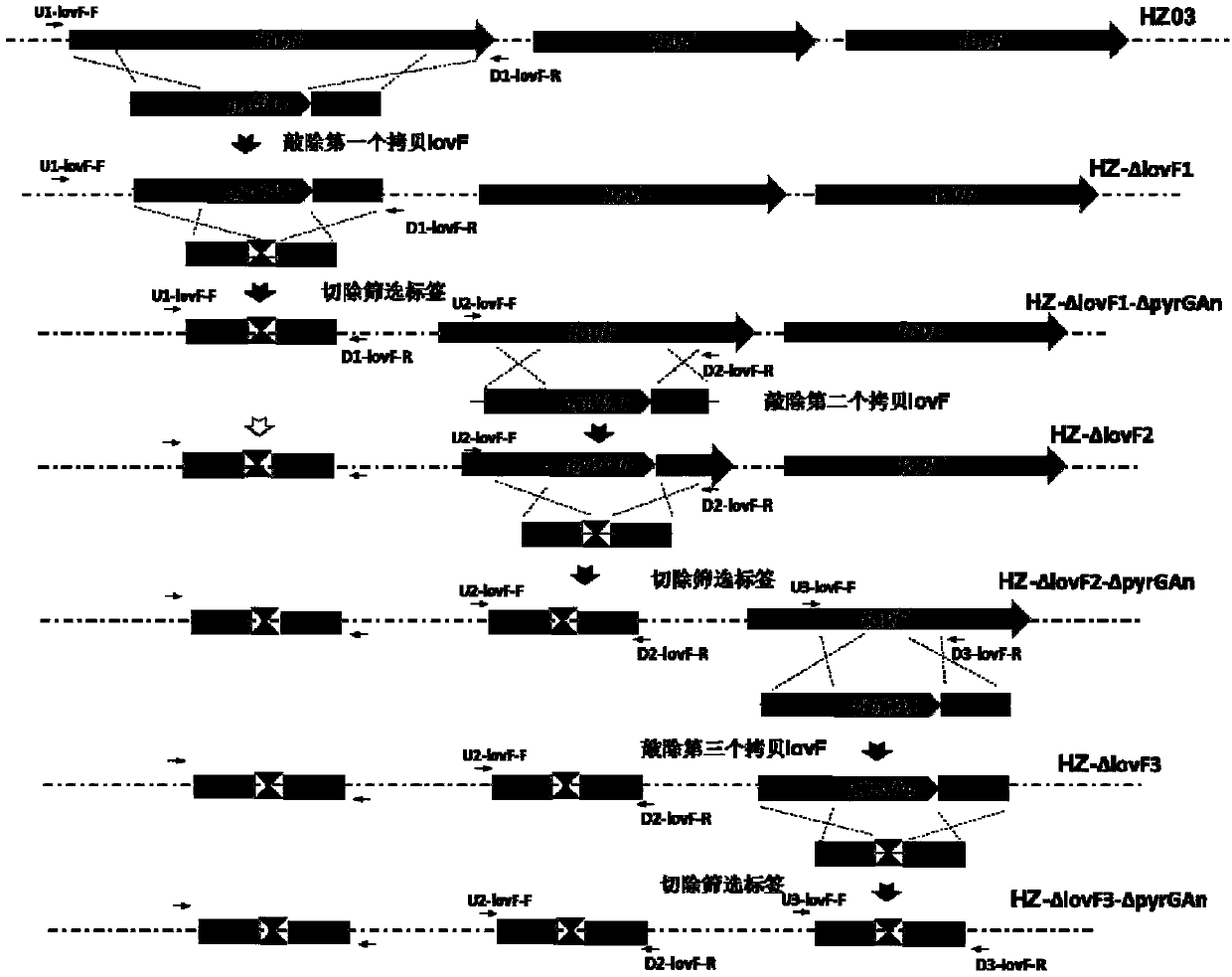
[0080] Knockout the lovF gene to complete the knockout of all copies, all primer positions and gene knockout strategy procedures are as follows figure 1 shown.
[0081] 1. Knockout of the first lovF copy
[0082] (1) Construction of lovF gene targeting element lovF-KO1
[0083] According to the genome information of Aspergillus terreus NIH2624 (GenBank:AAJN00000000.1) and the information of lovastatin synthesis gene cluster of Aspergillus HZ01, the following primers were designed and synthesized:
[0084] U1-lovF-F:5'-gctcccatagctatggttggc-3' (SEQ ID No.1);
[0085] U1-lovF-R: 5'-GTTCAATCATCTCTCCCTTAgagtcttcaagacgatcgcagc-3' (SEQ ID No. 2);
[0086] D1-lovF-F: 5'-CGTATTTCTCGCCTGTGTGatctgcgagtggctggtcgatc-3' (SEQ ID No. 3);
[0087] D1-lovF-R: 5'-gcatctcagaacgggatgctg-3' (SEQ ID No. 4).
[0088] Genomic DNA of Aspergillus HZ01 was used as a template, and pfu DNA polymerase (Fermentas, catalog number: EP0501) was used ...
Embodiment 3
[0144] Embodiment 3, the construction of overexpression-specific transcription regulator LovE bacterial strain
[0145] 1. Design and synthesize the following primers:
[0146] Uku80-F: 5'-agcacaaacatattgatcagc-3' (SEQ ID No. 31);
[0147] pyrGAn-R:5'-GGATCCTCCCAGAGTGTAAgcatcaaatcgtcgtaccgca-3' (SEQ ID No. 32);
[0148] TtrpC-F3:5'-aagcttgagatccacttaacgttactgaaatcatc-3' (SEQ ID No. 33);
[0149] Dku80-R: 5'-gaaggcgaaaagtagtctcgtg-3' (SEQ ID No. 34).
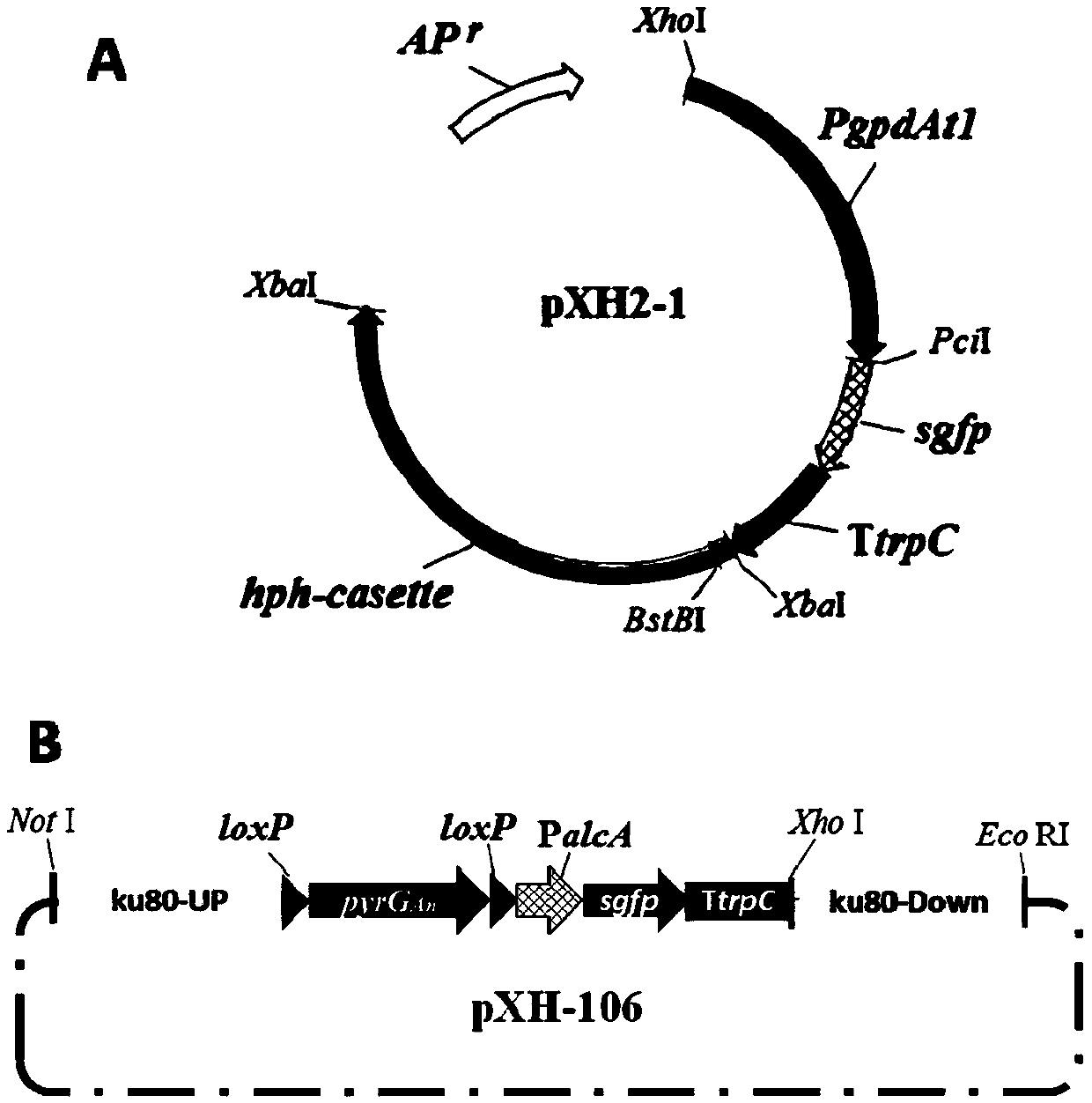
[0150] Using the plasmid pXH-106 as a template, PCR amplification was performed with primer pairs Uku80-F / pyrGAn-R and TtrpC-F3 / Dku80-R respectively to obtain the upstream homology arm Uku80-pyrGAn and the downstream homology arm TtrpC-Dku80.
[0151] 2. Design and synthesize the following primers:
[0152] lovE-F: 5'-ACAACTCATCAATCATCATCAC atggctgcagatcaaggtat-3' (SEQ ID No. 35);
[0153] lovE-R: 5'-GTTAAGTGGATCTCAAGCTTcatggaggaatattgttga-3' (SEQ ID No. 36).
[0154] Aspergillus HZ01 genome DNA was used as a template, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com