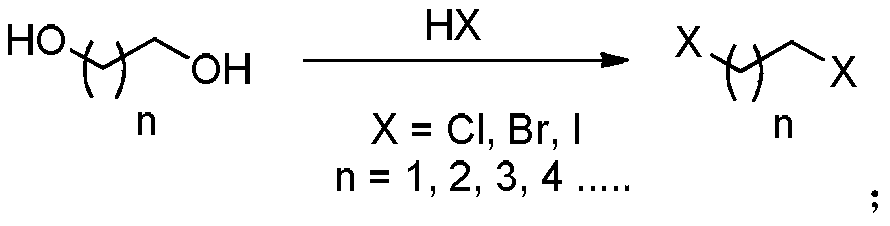Continuous method for preparation of dihalogenated alkane from diol compound
A technology for dihalogenated alkanes and compounds, which is applied in the field of continuity, can solve the problems of incompatibility with industrialized production, long reaction time, unsuitable for halogenated hydrocarbon production, etc., achieve less discharge of acidic tail gas and waste acid, and realize continuous and automated production, avoiding the effects of ringing and aggregation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
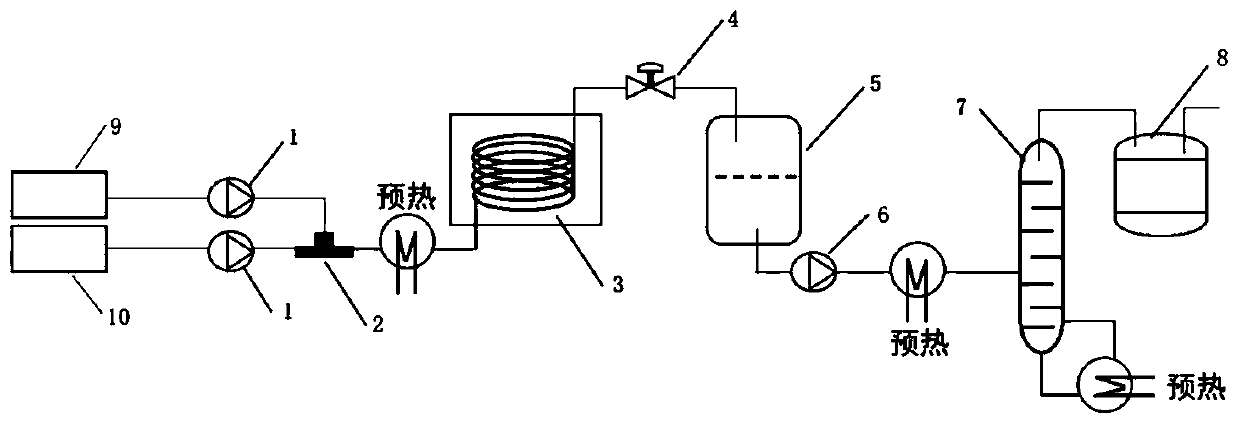
[0038] Reaction device: The reactor used in this embodiment is a chip reactor, which has an umbrella-shaped pulse variable diameter structure inside. The reaction residence time was calculated from the reactor liquid holdup and material flow rate data. The heat exchange medium is heat transfer oil.
[0039] Raw material tank 9 is equipped with 1,2-ethanediol, and raw material tank 10 is equipped with 35% concentrated hydrochloric acid, and these two kinds of raw materials are directly pumped into microreactor with 6.8mL / min and 35.3mL / min respectively through high pressure constant flow pump 1 respectively 3. To carry out the reaction, control the temperature of the reactor to 135° C., adjust the pressure of the back pressure valve 4 to 2.5 MPa, and the reaction residence time to 9.5 min. The milky reaction solution is cooled to 40°C by a coil tube and then enters a static separatory tank for 5 separate liquids. The organic phase in the lower layer enters the rectification to...
Embodiment 2
[0045] Reaction device: The reactor used in this embodiment is a chip-type microreactor, which has an umbrella-shaped pulse variable diameter structure inside. The reaction residence time was calculated from the reactor liquid holdup and material flow rate data. The heat exchange medium is heat transfer oil.
[0046]Raw material tank 9 is equipped with 1,3-glycerol, and raw material tank 10 is equipped with 35% concentrated hydrochloric acid, and these two kinds of raw materials are pumped into mixer 2 with 5.3mL / min and 24.1mL / min respectively through high pressure constant flow pump 1 respectively , after the temperature of the material reaches 100°C through the tubular preheating module, it enters the microreactor 3 for reaction. The temperature of the reactor is controlled at 140°C, the pressure of the back pressure valve 4 is adjusted to 3.0MPa, and the reaction residence time is 11.7min. The milky reaction solution is cooled to 70°C by a coil tube and then enters a stat...
Embodiment 3
[0052] Reaction device: The reactor used in this embodiment is a chip-type microreactor, which has an umbrella-shaped pulse variable diameter structure inside. The reaction residence time was calculated from the reactor liquid holdup and material flow rate data. The heat exchange medium is heat transfer oil.
[0053] Raw material tank 9 is equipped with 1,4-butanediol, and raw material tank 10 is equipped with 35% concentrated hydrochloric acid, and these two kinds of raw materials are respectively pumped into micro-mixer 2 with 5.9mL / min and 20.3mL / min through high-pressure constant flow pump 1 respectively , after the temperature of the material reaches 120°C through the tubular preheating module, it enters the microreactor 3 for reaction, the temperature of the reactor is controlled at 170°C, the pressure of the back pressure valve 4 is adjusted to 3.0MPa, and the reaction residence time is 15.3min. The milky reaction solution is cooled to 70°C by a coil, and then enters a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com