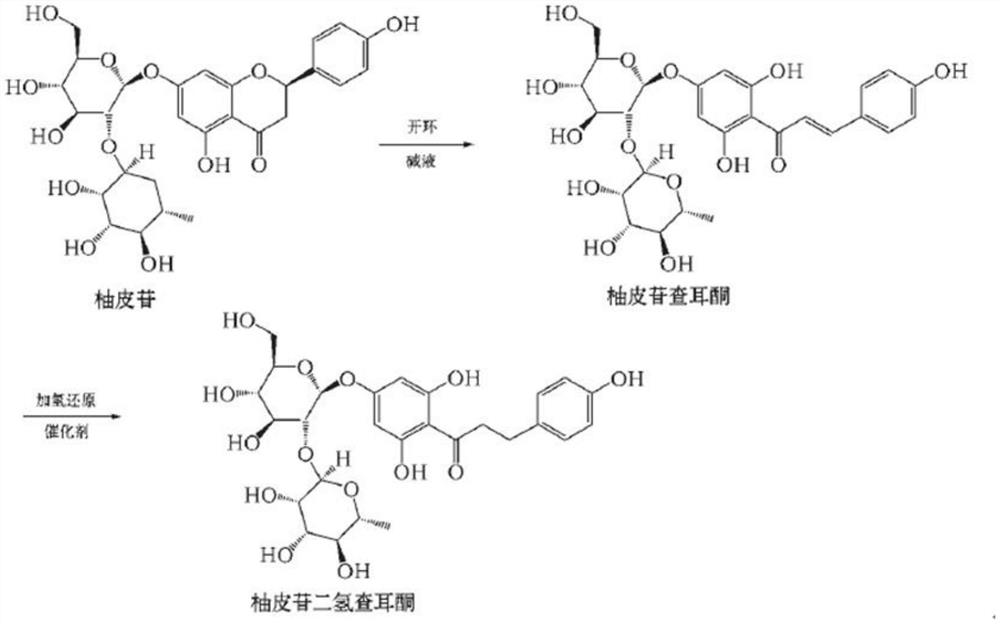
A kind of synthetic method of naringin dihydrochalcone
A technology of naringin dihydrochalcone and a synthesis method is applied in the field of synthesis of intermediate product naringin dihydrochalcone, and can solve the problem that no safe, non-toxic phloridzin synthesis process, toxic reaction, load It can solve the problem of high volume, and achieve the effects of easy access, environmental protection of solvents, and rich sources.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] A kind of synthetic method of naringin dihydrochalcone, is characterized in that, comprises the following steps:
[0036] 1) Preparation of crude wet product of naringin dihydrochalcone
[0037] 1.1) 10g of raw material naringin was dissolved in 100g of 5% sodium hydroxide solution, then 0.7g of Raney nickel catalyst was added, placed at room temperature, hydrogenation reaction was carried out under 0.5Mp hydrogen pressure for 1.5h, liquid Monitor the reaction until the content of naringin dihydrochalcone is 97-98%, and stop the reaction to obtain a reaction liquid;
[0038] 1.2) Filter the reaction liquid obtained in step 1), remove the catalyst, cool down to 0-5°C, and add 12g of concentrated hydrochloric acid to adjust the pH of the reaction liquid to 4-5; then raise the temperature to 5-10°C, keep it warm for 4 hours, and use a plate and frame press Filtration, obtained naringin dihydrochalcone thick wet product 19g;
[0039] 2) Preparation of naringin dihydrochal...
Embodiment 2
[0043] A kind of synthetic method of naringin dihydrochalcone, is characterized in that, comprises the following steps:
[0044] 1) Preparation of crude wet product of naringin dihydrochalcone
[0045] 1.1) 50g of raw material naringin was dissolved in 500g of 5% sodium hydroxide solution, then 3.5g of Raney nickel catalyst was added, placed at room temperature, hydrogenation reaction was carried out under 0.5Mp hydrogen pressure for 1.5h, liquid Monitor the reaction until the content of naringin dihydrochalcone is 97-98%, and stop the reaction to obtain a reaction liquid;
[0046]1.2) Filtrate the reaction solution obtained in step 1), remove the catalyst, cool down to 0-5°C, and add 60g of concentrated hydrochloric acid to adjust the pH of the reaction solution to 4-5; then heat up to 5-10°C, keep it warm for 4 hours, and then use a plate and frame press Filter, and make naringin dihydrochalcone rough wet product 98g;
[0047] 2) Preparation of naringin dihydrochalcone bou...
Embodiment 3
[0051] A kind of synthetic method of naringin dihydrochalcone, is characterized in that, comprises the following steps:
[0052] 1) Preparation of crude wet product of naringin dihydrochalcone
[0053] 1.1) Dissolve 30g of raw material naringin into 300g of 5% sodium hydroxide solution, then add 2.1g of Raney nickel catalyst, place at room temperature, carry out hydrogenation reaction under 0.5Mp hydrogen pressure for 1.5h, liquid Monitor the reaction until the content of naringin dihydrochalcone is 97-98%, and stop the reaction to obtain a reaction liquid;
[0054] 1.2) Filter the reaction liquid obtained in step 1), remove the catalyst, cool down to 0-5°C, and add 36g of concentrated hydrochloric acid to adjust the pH of the reaction liquid to 4-5; then raise the temperature to 5-10°C, keep it warm for 4 hours, and use a plate and frame press Filter to obtain naringin dihydrochalcone rough wet product 59g;
[0055] 2) Preparation of naringin dihydrochalcone boutique
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com