A method for removing chloride ions in chlorinated sulfuric acid solution by using fluidized bed electrodes
A fluidized bed, chlorosulfuric acid technology, applied in the fields of chemical industry, metallurgy and environmental protection, can solve the problem of low electrosorption absorption, and achieve the effects of improving selectivity, high economic efficiency, high economic efficiency and environmental protection benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
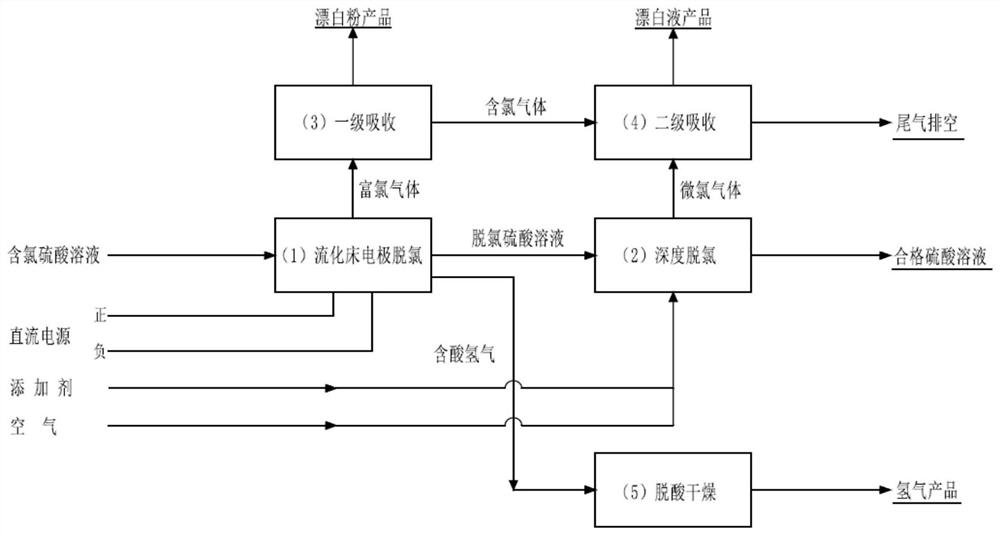
[0034] figure 1 It is a schematic flow chart of a method for removing chloride ions in a chlorine-containing sulfuric acid solution by using a fluidized bed electrode according to the present invention.
[0035] combine figure 1 , a method for removing chloride ions in a chlorinated sulfuric acid solution using a fluidized bed electrode used in this embodiment, said method comprising a fluidized bed electrode dechlorination process 1, a deep dechlorination process 2, and a primary absorption process 3 , secondary absorption process 4 and deacidification drying process 5 five processes, specifically according to the following steps:
[0036] 1) The chlorine-containing sulfuric acid solution is sent to the fluidized bed electrode dechlorination process, and under the action of direct current, high-efficiency electrochemical oxidation is realized to obtain acid hydrogen gas, chlorine-rich gas and dechlorinated sulfuric acid solution;
[0037] 2) The acid-containing hydrogen is ...
Embodiment 2
[0042] This embodiment uses the method described in Example 1 for removing chloride ions in a chlorinated sulfuric acid solution using a fluidized bed electrode. . The fluidized bed electrode dechlorination process 1 adopts a fluidized bed electrode reactor, adopts a granular anode with a diameter of 20 μm, and an anode current density of 5 A / m 2 , the anode is made of lead alloy; the cathode is plate-shaped, made of stainless steel, and the cathode current density is 50A / m 2 . In the fluidized bed electrode dechlorination process 1, the operating linear velocity is 0.001 m / s, the temperature is 20° C., the bed expansion ratio is 1.1, the voltage is 1.5 V, and the current efficiency is 15%. In the deep dechlorination step 2, the additive is hypochlorous acid, and the dosage is 1.0 times of the amount of residual chlorine, and the concentration of chloride ions in the obtained qualified sulfuric acid solution is within 50 mg / L. In the primary absorption process 3, the absorb...
Embodiment 3
[0044] This embodiment uses a method for removing chloride ions in the chlorine-containing sulfuric acid solution described in Example 1 by using a fluidized bed electrode. The concentration of chloride ions in the chlorine-containing sulfuric acid solution is 10 g / L, and the concentration of sulfuric acid is 5.0M. The fluidized bed electrode dechlorination process 1 adopts a fluidized bed electrochemical reactor, adopts a granular anode with a diameter of 3.0 cm, and an anode current density of 500 A / m 2 , the anode material is titanium-based ruthenium dioxide coating; the cathode is rod-shaped, the material is nickel-based alloy, and the cathode current density is 10000A / m 2 . In the fluidized bed electrode dechlorination process 1, the operating linear velocity is 10m / s, the bed expansion ratio is 3.8, the temperature is 50°C, the voltage is 4.5V, and the current efficiency is 75%. In the deep dechlorination step 2, the additive is hypochlorous acid, and the dosage is 1.1 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com