Indirect background fluorescence colloidal gold immunochromatographic test strip based on double-labeled signal amplification and application thereof
A colloidal gold, double-labeled technology, applied in the fields of food safety detection and biomedicine, can solve the problems of matrix interference, sensitivity needs to be improved, repeatability is not optimistic, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
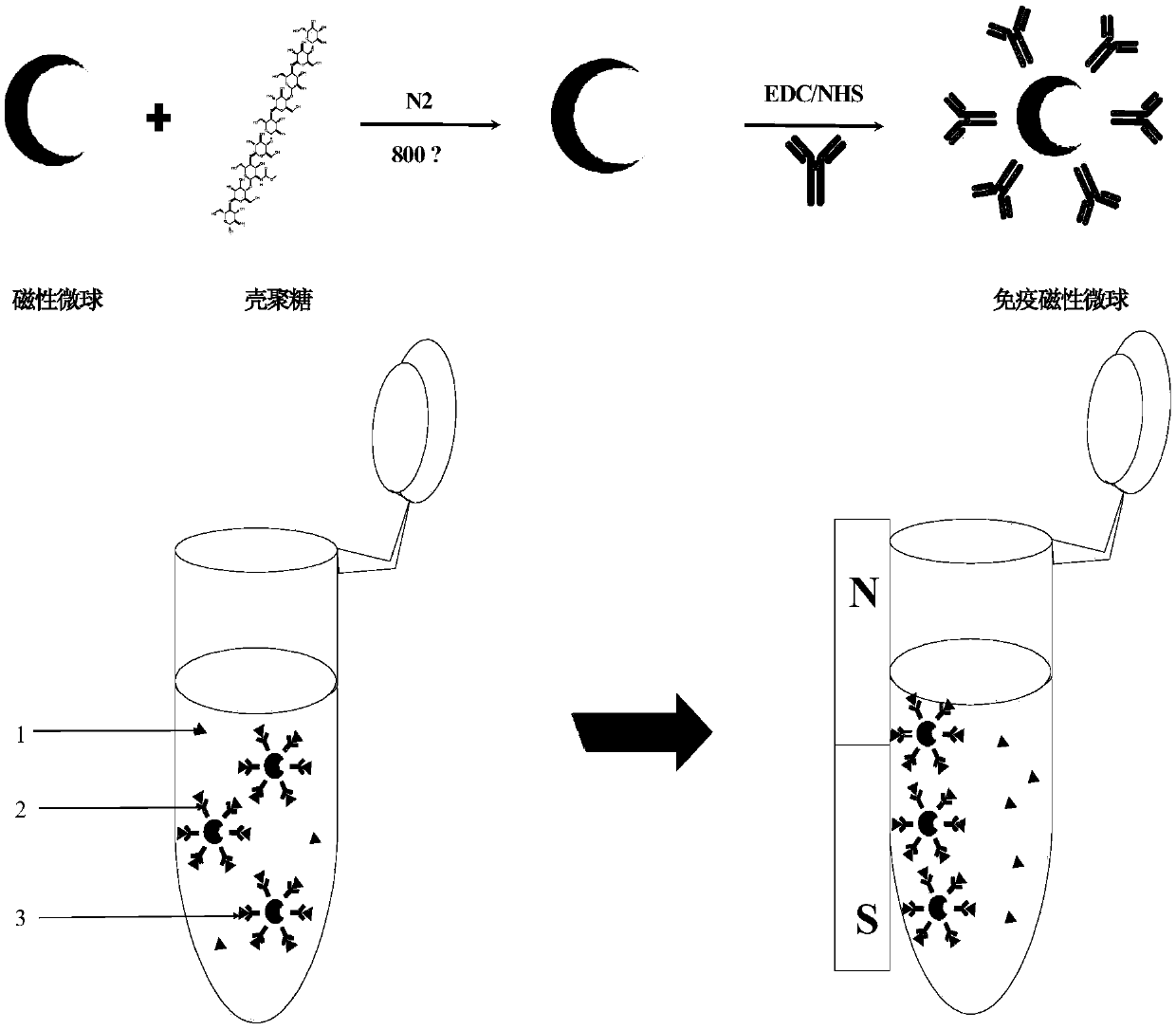
[0090] Example 1: Preparation of immunomagnetic microspheres and its application in enriching clenbuterol hydrochloride in swine urine samples
[0091] 1. Preparation of immunomagnetic microspheres
[0092] 1. Preparation of Suspension A
[0093] Weigh 0.1g Fe 3 o 4 Add 20 mL of distilled water to the magnetic microspheres, and form suspension A after ultrasonication for 30 min.
[0094] 2. Preparation of Suspension B
[0095] 0.8g chitosan (purchased from Bailingwei Technology Co., Ltd., degree of deacetylation: 95%) was dissolved in 15mL of dilute acetic acid solution with a volume fraction of 2%, to obtain a homogeneous suspension B with a mass fraction of 2%.
[0096] 3. Fe 3 o 4 @Preparation of chitosan magnetic microspheres
[0097] Mix suspension A and suspension B according to the volume ratio of 4:1, stir for 1 h, spray dry by spray dryer for 24 h, and then calcinate at 800 °C for 4 h under nitrogen to obtain Fe 3 o 4 @Chitosan magnetic microspheres.
[0098...
Embodiment 2
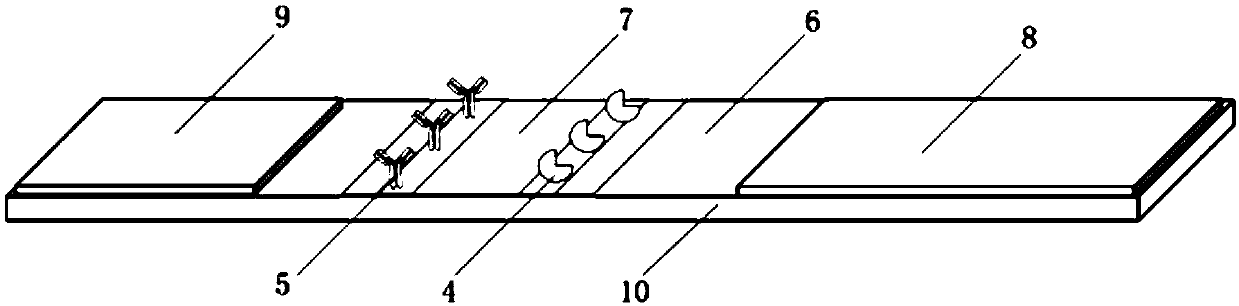
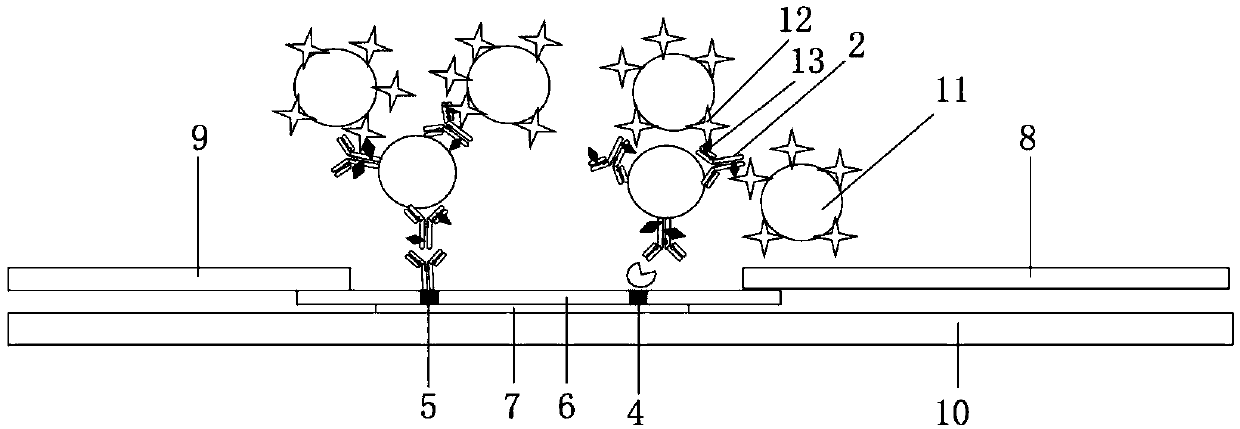
[0110] Example 2: Preparation of double-labeled indirect background fluorescent colloidal gold test strips
[0111] 1. Preparation of double-labeled colloidal gold
[0112] 1. Preparation of colloidal gold-clenbuterol hydrochloride monoclonal antibody-biotin (GNPs-clenbuterol hydrochloride antibody-biotin)
[0113] (1) Preparation of biotinylated clenbuterol monoclonal antibody solution
[0114] Aminated biotin (purchased from SIGMA Company) with a concentration of 3.8 mg / ml was added to 1 mg of clenbuterol hydrochloride monoclonal antibody, and after rotating for 1 hour, dialyzed three times with a dialysis membrane (8k MWCO), buffer (pH7 .4) The formula is as follows: 50mM PB, 75mM NaCl, each time 2L of dialysis for more than 3h. Then use a pipette to carefully suck out the biotinylated antibody from the dialysis bag, adjust the concentration of the biotinylated antibody with a 50 mM PBS solution, and obtain a biotinylated clenbuterol monoclonal antibody with a concentrati...
Embodiment 3
[0129] Embodiment 3: Quantitative and qualitative detection of clenbuterol hydrochloride
[0130] 1. Qualitative detection method of clenbuterol hydrochloride
[0131] 1. Enrich and purify the sample to be tested using the immunomagnetic microspheres in Example 1 to obtain a processed sample.
[0132] 2. Combine 150 μL of the treated sample with 3 μL of the GNPs-clenbuterol hydrochloride antibody-biotin solution prepared in step 1 of Example 2 and 3 μL of GNPs-streptavidin prepared in step 1 of Example 2 The solution was mixed and incubated at room temperature for 3 min to obtain a mixed solution.
[0133] 3. Take 120 μL of the mixed solution and drop it on the sample pad of the test strip. Qualitative observation with naked eyes after 10 min.
[0134] If both the C line and the T line are red, the sample to be tested does not contain clenbuterol hydrochloride;
[0135] If the C line is red and the T line is not, the sample to be tested contains clenbuterol hydrochloride. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| coefficient of variation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com