Preparation methods of transition metal carbide, carbon material and transition metal chalcogenide and application of transition metal chalcogenide
A technology of chalcogenides and transition metals, which is applied in the preparation of carbon materials, transition metal chalcogenides, and transition metal carbides, can solve the problems of poor rate capability, short cycle life, and fast capacity decay of the tested battery, and achieve Strong adsorption energy, extended cycle life, and reduced swelling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] A preparation method of transition metal carbides, comprising the steps of:
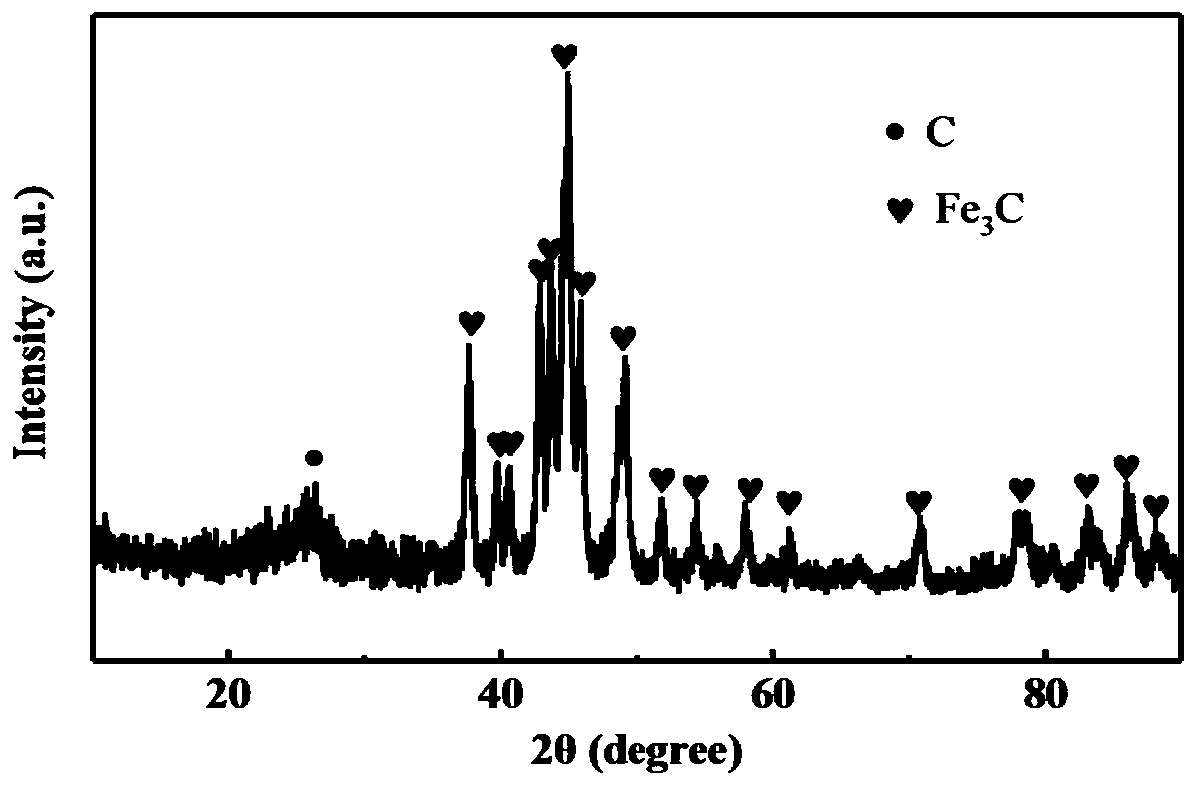
[0032] Disperse 1g of polyvinylpyrrolidone and 1g of ferric nitrate hexahydrate in deionized water, stir at 25°C for 2h to obtain a uniform solution, and then put the solution in a drying oven at 60°C for 24h to obtain a colloidal precursor. Put it into a corundum boat, pass Ar protective atmosphere in a tube sintering furnace, and sinter at 700 ° C for 3 hours to obtain iron carbide.
[0033] The above-mentioned iron carbide is used to prepare carbon materials, and the specific preparation method is as follows:
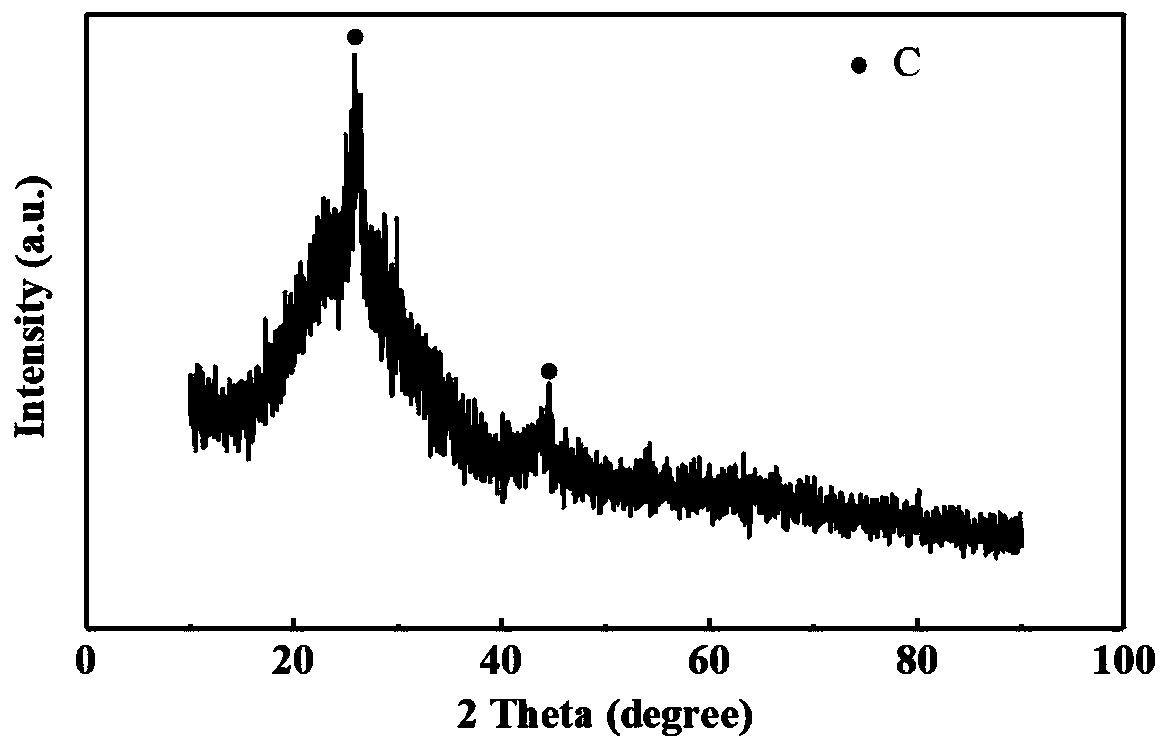
[0034] The iron carbide was pickled with 1M hydrochloric acid, washed with deionized water, and dried to obtain a porous carbon material. The porous carbon material can be used in lithium-ion batteries.
[0035] The above-mentioned iron carbide is used to prepare iron chalcogen compounds, and the specific preparation method is as follows:
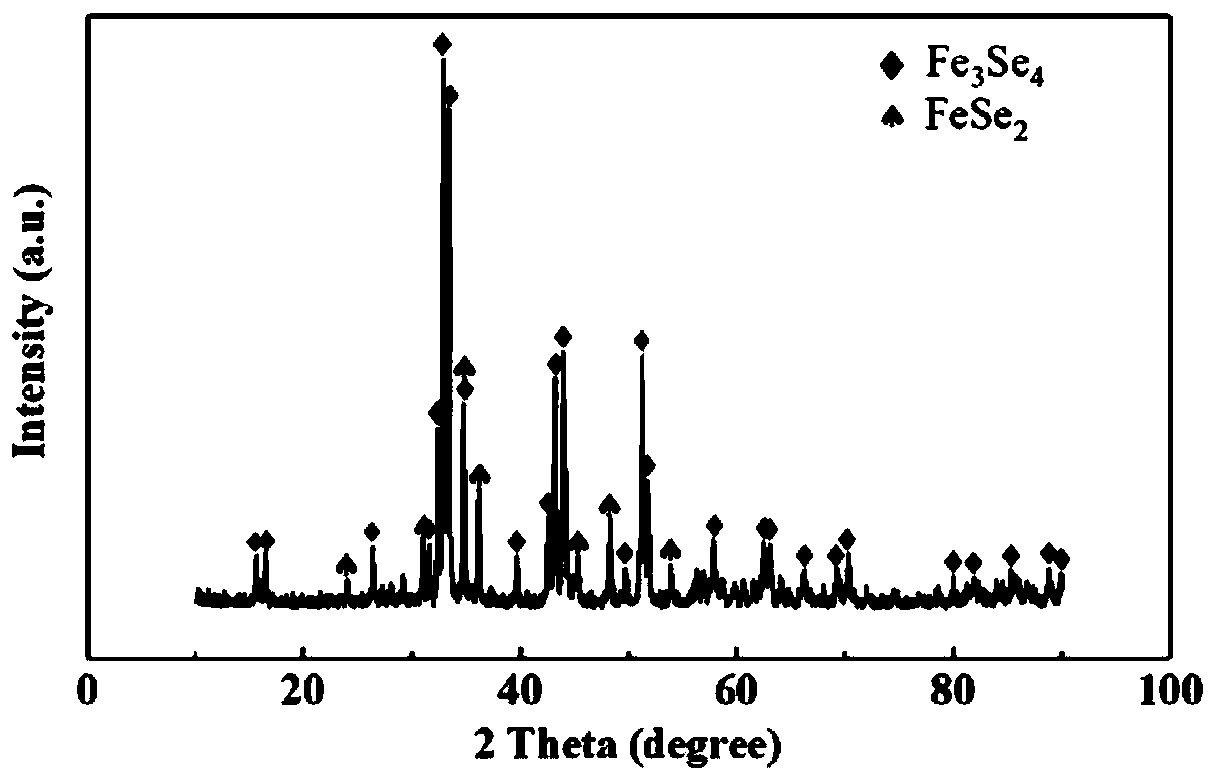
[0036] 1g of iron carbide and 1g of sele...
Embodiment 2
[0038] A preparation method of transition metal carbides, comprising the steps of:
[0039] Disperse 9 g of agar gel and 5 g of cobalt nitrate hexahydrate in deionized water, stir at 45 ° C for 1 h to obtain a uniform solution, then put the solution in a drying oven at 70 ° C for 18 h to obtain a colloidal precursor, put the precursor in Put it into a corundum boat, pass Ar protective atmosphere in a tubular sintering furnace, and sinter at 900 ° C for 1 hour to obtain cobalt carbide.
[0040] The above-mentioned cobalt carbide is used to prepare carbon materials, and the specific preparation method is as follows:
[0041] The cobalt carbide was pickled with 1M sulfuric acid, washed with deionized water, and dried to obtain a porous carbon material. The porous carbon material can be used in lithium-ion batteries.
[0042] The above-mentioned cobalt carbide is used to prepare cobalt chalcogenides, and the specific preparation method is as follows:
[0043] Grind and mix 2g o...
Embodiment 3
[0045] A preparation method of transition metal carbides, comprising the steps of:
[0046] Disperse 3g of polyvinylpyrrolidone and 9g of copper nitrate hexahydrate in deionized water, stir at 30°C for 3h to obtain a uniform solution, and then put the solution in a drying oven at 90°C for 12h to obtain a colloidal precursor. Put it into a corundum boat, pass through an Ar protective atmosphere in a tubular sintering furnace, and sinter at 500 ° C for 5 hours to obtain copper carbide.
[0047] The above-mentioned copper carbide is used to prepare carbon materials, and the specific preparation method is as follows:
[0048] The copper carbide was pickled with 1M nitric acid, washed with deionized water, and dried to obtain a porous carbon material. The porous carbon material can be used in lithium-ion batteries.
[0049] The above-mentioned copper carbide is used to prepare copper chalcogenides, and the specific preparation method is as follows:
[0050]Grind and mix 3g of ir...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Discharge specific capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com