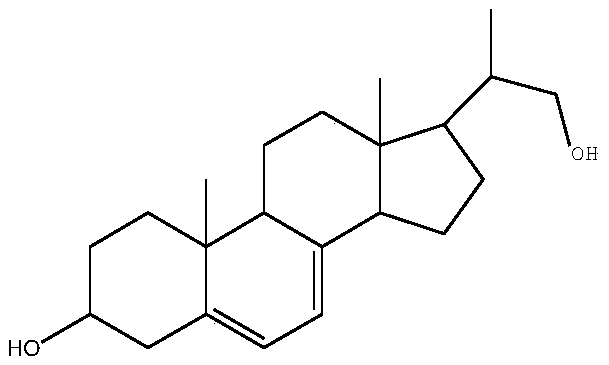
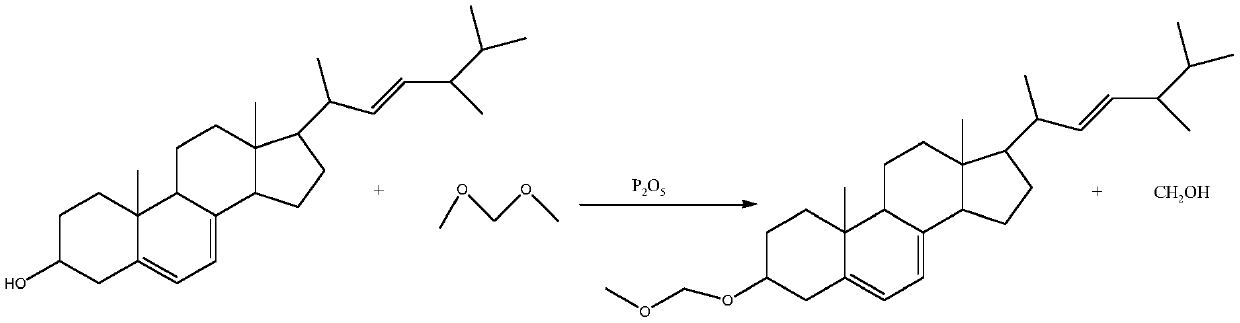
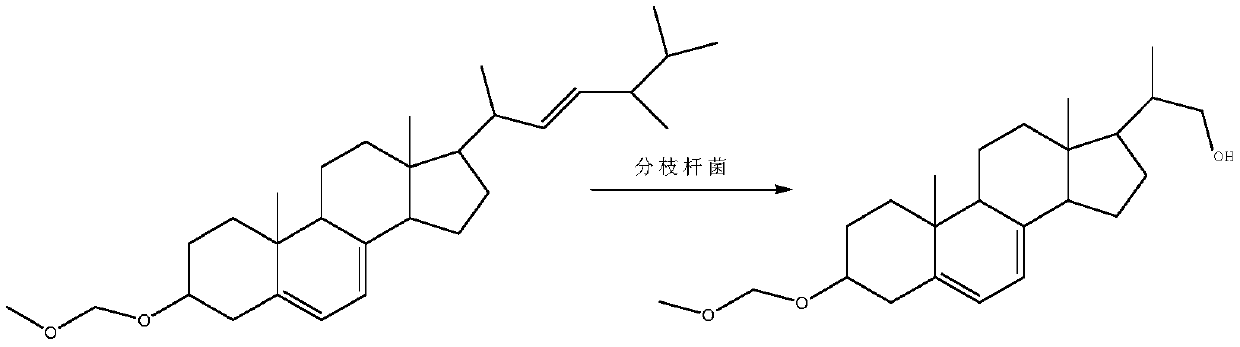
Method for preparing intermediate by using resting cell biological fermentation of ergosterol etherate
A technology of ergosterol and biological fermentation, applied in biochemical equipment and methods, microorganism-based methods, treatment of microorganisms with electricity/wave energy, etc. Problems such as low yield, to achieve the effect of improved reaction yield, low cost and short route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1 strain mutagenesis
[0036] Starting strain: Mycobacterium sp.B-NRRL 3683
[0037] (1) Strain culture:
[0038] Solid slant medium: M1+2% agar.
[0039] Liquid seed medium: M1.
[0040] Cultivate the strain B-NRRL 3683 in a solid slant medium, connect a ring of well-grown slant seeds and activate in a 500ml Erlenmeyer flask containing 100ml liquid seed medium, shake and culture at 200rpm at 30°C for 48h; take the activated slant seeds 10ml of the primary liquid seeds were inserted into a 500ml Erlenmeyer flask containing 100ml of liquid seed medium for secondary seed cultivation, and cultured at 30°C and 200rpm on a shaker for 48h.
[0041] (2) Bacterial suspension preparation
[0042] Put the grown secondary seeds into a 10ml centrifuge tube and centrifuge at 10000rpm for 5min, discard the supernatant, collect the thallus, wash twice with the potassium phosphate buffer solution of pH6.0, and then use sterile potassium phosphate buffer solution (pH6 .0) ...
Embodiment 2
[0048] Embodiment 2 Seed culture
[0049] Strain name: Mycobacterium sp.B-NRRL 3683 mutagenic strain
[0050] (1) Incline cultivation
[0051] Formula: peptone 0.1-10g / L, yeast extract 0.1-10g / L, glucose 0.1-10g / L, disodium hydrogen phosphate 0.1-10g / L, agar 20g / L, pH=7.5-8.0.
[0052] Sterilize at 121°C for 30 minutes. After solidification and molding, inoculate under sterile conditions.
[0053] After inoculation, culture at 30°C for 4 days, and store in a refrigerator at 4°C for no more than 1 month.
[0054] (2) Shake flask seed culture
[0055] Formula: peptone 0.1-10g / L, yeast extract 0.1-10g / L, glucose 0.1-10g / L, disodium hydrogen phosphate 0.1-10g / L, pH=7.5-8.0.
[0056] Sterilize at 121°C for 30 minutes. Cool to room temperature.
[0057] 1. Primary seed culture
[0058] Inoculate under sterile conditions, inoculum volume: scrape 1 ring per 100ml. After inoculation, culture at 30°C and 200rpm shaker for 48h.
[0059] 2. Secondary seed culture
[0060] Inocu...
Embodiment 3
[0065] Embodiment 3 Ergosterol 3-position etherification protection
[0066] Ratio of materials: 1500g of methylal, 100g of ergosterol, 100g of diatomaceous earth, 50g of phosphorus pentoxide, 4g of sodium carbonate (used as 1% aqueous solution), 200g of water.
[0067] Add ergosterol and methylal in proportion to the reaction bottle, heat up to 25°C, stir until completely dissolved, add diatomaceous earth, then slowly add phosphorus pentoxide, control the temperature during the addition process not to exceed 30°C, around 25°C Stir for 1-1.5h, the reaction is complete as detected by thin-layer chromatography, heat up to above 30°C, filter while hot, wash the filter cake and reaction bottle with a small amount of water, and dry at 50°C. A light yellow solid was obtained, which was dried in an oven at 40-50° C. to a constant weight of 118.4 g, thus obtaining the crude ergosterol ether compound.
[0068] Add 2 times the volume of acetone to the crude etherified product obtained,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com