Preparation method of aprepitant intermediate impurity
A technology for aprepitant and intermediates, applied in the field of preparation of aprepitant intermediate impurities, can solve the problems of low repetition rate and yield, complex ethanol synthesis route, etc., and achieve the effect of simple equipment and environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
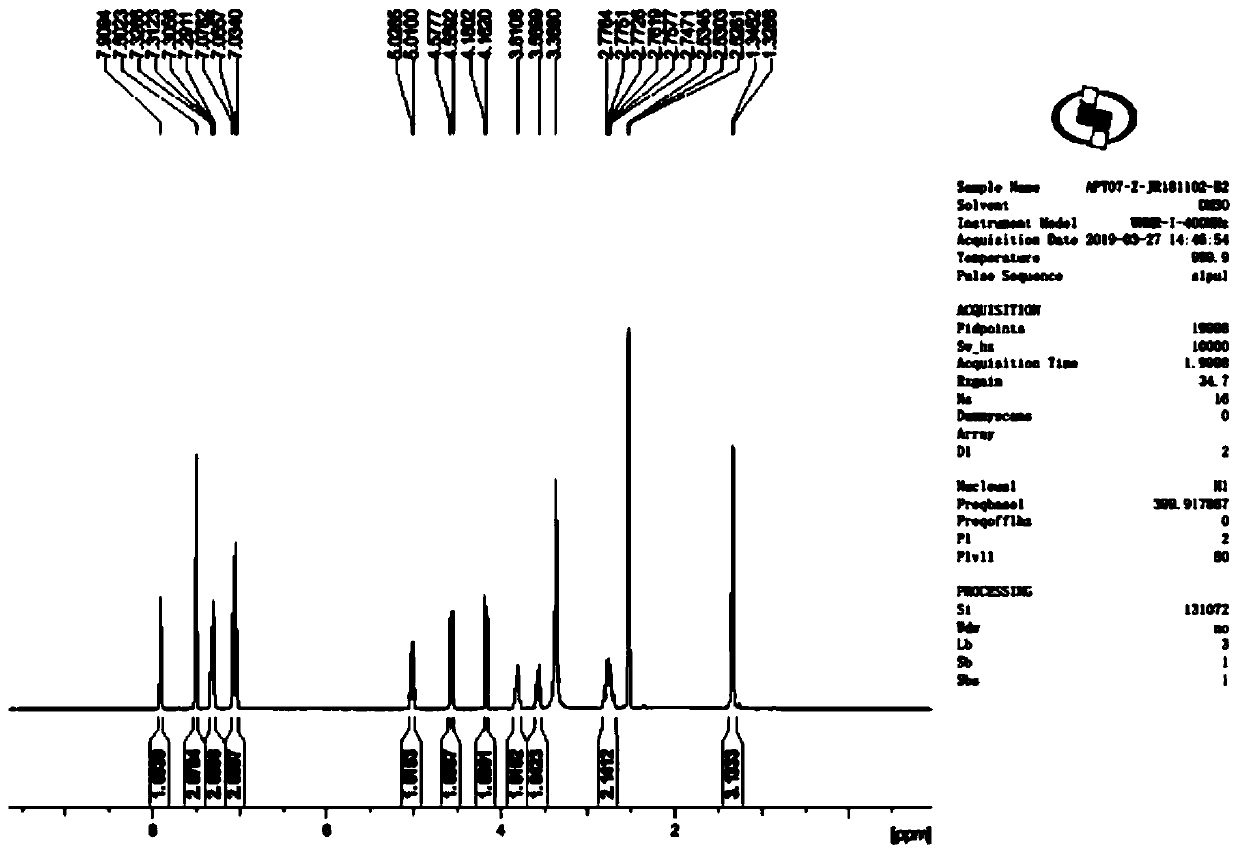
Image
Examples
preparation example Construction
[0031] The preparation method comprises the following reaction steps:
[0032] (A) carry out Grignard reaction with raw material 1 and Grignard reagent 4-fluorophenylmagnesium halide;
[0033] (B) reducing the reaction product obtained in step (A) with a reducing agent to obtain compound 4;
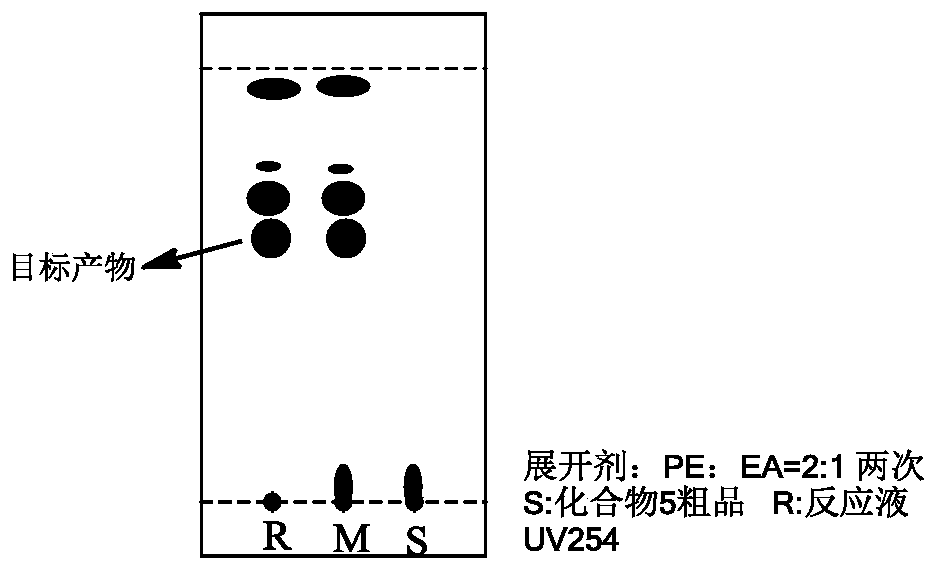
[0034] (C) debenzylation reaction of obtained compound 4 with a deprotection reagent to obtain crude product 5;
[0035] (D) reacting the obtained crude product 5 with a protecting reagent in the presence of a solvent and a base to obtain compound 6;
[0036] (E) deprotecting the obtained compound 6 with an acid or a base in the presence of a solvent, and then obtaining the refined product 5 after separation and purification;
[0037] The protecting reagent is selected from: fluorenylmethoxycarbonyl chloride, benzyloxycarbonyl chloride or Boc-anhydride.
[0038] In some of these embodiments, the base in reaction (D) is at least one of triethylamine, N,N-diisopropylethylamine, potassium...
Embodiment 1
[0070]
[0071] (A) Add 50g of APT04 (raw material 1) and 50mL of THF into a 500mL three-necked flask, stir to dissolve and cool down to about 10°C in an ice-water bath under the protection of nitrogen. Add 168mL (1mol / L) of p-fluorophenylmagnesium bromide Grignard reagent dropwise at a temperature of 10-20°C. After the addition is complete, stir and react at a constant temperature of 5-20°C for 0.5h. After the reaction is monitored by TLC, the reaction system Slowly drop into 190mL of cold methanol to quench, and control the temperature below 5°C.
[0072] (B) Cool the obtained quenched reaction system to below 0°C, then add 8.5 g of sodium borohydride in batches, stir for 10 min, remove the ice-water bath, and let the temperature naturally rise to room temperature for the reaction. After 0.5 h, the reaction was monitored by TLC until complete. Concentrate the above system under reduced pressure at 48-50°C to dryness, add 300mL of ethyl acetate and 900mL of water, wash th...
Embodiment 2
[0077] The difference between this embodiment and Example 1 is that in reaction (D), the protective reagent is selected Boc-anhydride Instead of Fmoc-Cl.
[0078] (D) After dissolving 5 g of the crude oily product of Compound 5 obtained in step (C) of Example 1 with 70 mL of dioxane, add 6.2 mL of DIPEA and lower the temperature to -5 to 0°C, and add Boc- Acid anhydride 2.5g, after the dropwise addition, sample TLC to monitor the progress of the reaction. After the reaction, add 50mL of water and then add 80mL of DCM for extraction, let stand to separate layers, wash the organic phase with 50mL of saturated saline and concentrate to dryness to obtain compound 6. Crude sticky substance 8g. Petroleum ether-petroleum ether: ethyl acetate = 4:1 was used as the eluent to separate by silica gel column chromatography to remove small polar impurities, and further obtain 6 g of the crude product of compound 5 protected by the amino group. Take 6 g of the obtained crude product, put ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com