Primer group, kit and library construction method for detecting five bloodstream infection pathogens,
A technology of pathogens and primer sets, applied in the direction of microorganism-based methods, biochemical equipment and methods, microorganisms, etc., can solve the problems of increased signal detection load, low scalability, poor scalability, etc., to reduce non-specific amplification , avoid false positives, and reduce testing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
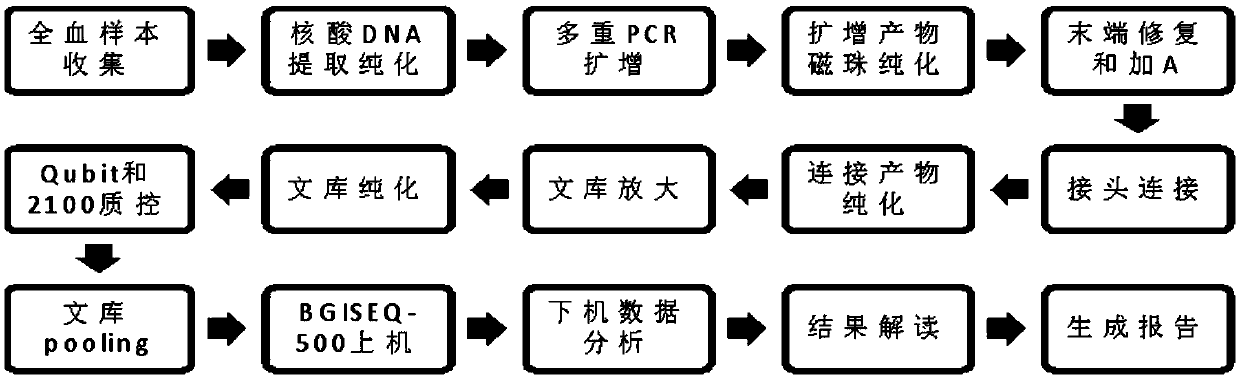
[0073] Select clinical blood culture positive samples and negative whole blood samples and R10 and R11 negative and positive control substances (as shown in Table 2) for testing, a total of 10 samples.
[0074] 1) Sample pretreatment and nucleic acid extraction:
[0075] Take 450 μL whole blood sample and add it to a 2.0 mL centrifuge tube pre-installed with 250 μL 0.5mm glass beads, seal the tube opening with a parafilm, shake at room temperature for 20 minutes, remove the sealing film after the shaking is completed, and put the centrifuge tube into a centrifuge for centrifugation at 8000rpm 30s, use a pipette to carefully draw 200 μL of the whole blood sample after breaking the wall for nucleic acid extraction. The nucleic acid extraction process was performed according to the instructions of the MagPureBlood DNA KF Kit, and the final melting volume of the nucleic acid was 40 μL.
[0076] 2) Extract nucleic acid purification
[0077] Purify the extracted nucleic acid with 1 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com