Difunctional self-healing polymer electrolyte and preparation method thereof
A self-healing, polymer technology, applied in the direction of circuits, electrical components, secondary batteries, etc., can solve the problems of difficult to meet expectations, weak binding force, and the performance of lithium-ion batteries is not as good as before, and achieve self-healing effect improvement , Electrochemical performance improvement, good self-healing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] The present invention also provides a method for preparing a bifunctional self-healing polymer electrolyte, comprising the following steps:
[0037] S1: react the monomer with double bond and the monomer with disulfide bond in the first solution, and obtain the cross-linking agent with disulfide bond through nucleophilic addition reaction;
[0038] S2: Dissolve the cross-linking agent with disulfide bond obtained in S1 and polyethylene glycol with double bond in the second solvent, add initiator and chain transfer reagent, after deoxygenation, heat the reaction to obtain PEG-SS copolymer;
[0039] S3: Dissolving the PEG-SS copolymer obtained in S2 in a third solvent, adding lithium salt and stirring evenly, casting the mixed solution to form a film, and drying to obtain a bifunctional self-healing polymer electrolyte.
[0040] Wherein, the first solvent is one or more of tetrahydrofuran, dimethyl sulfoxide, N,N-dimethylformamide, and N-methylpyrrolidone, and the second...
Embodiment 1
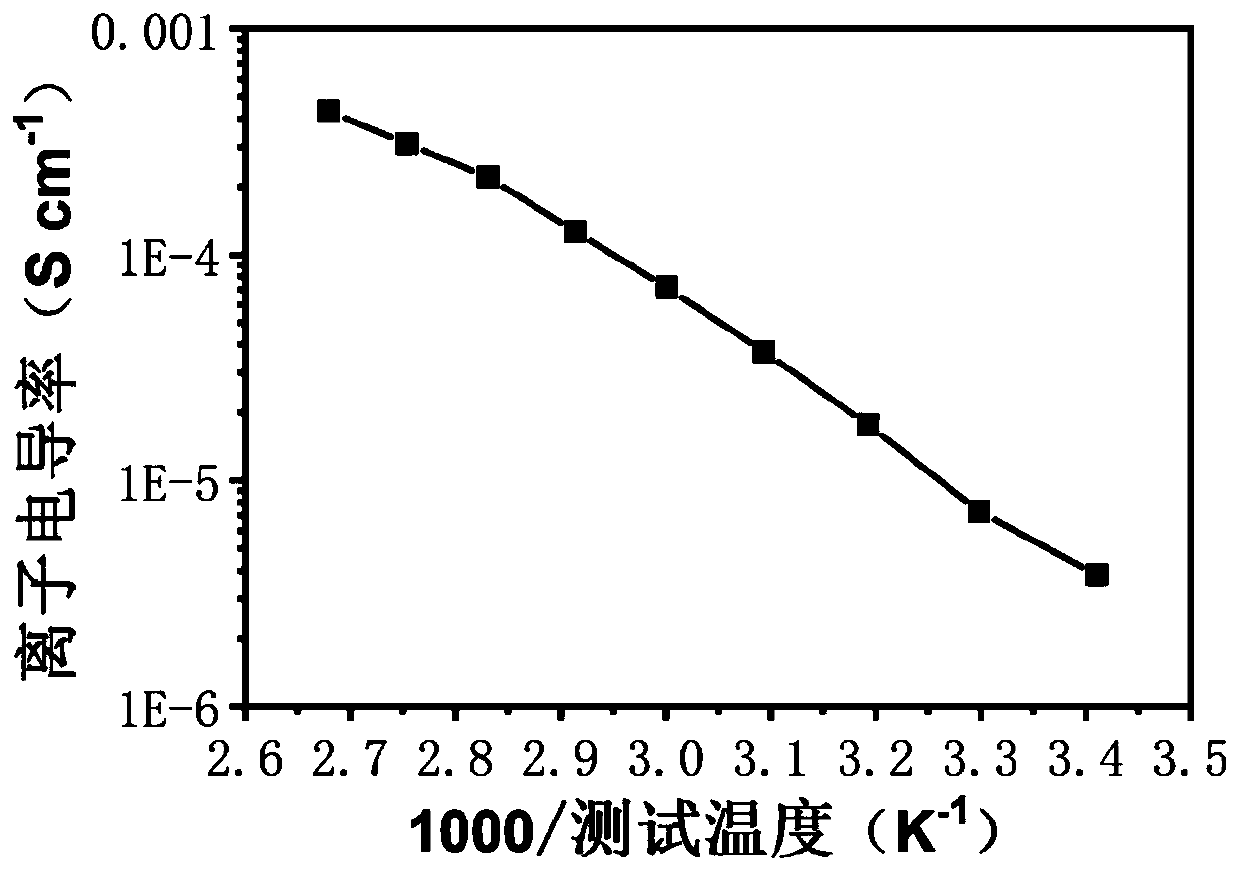
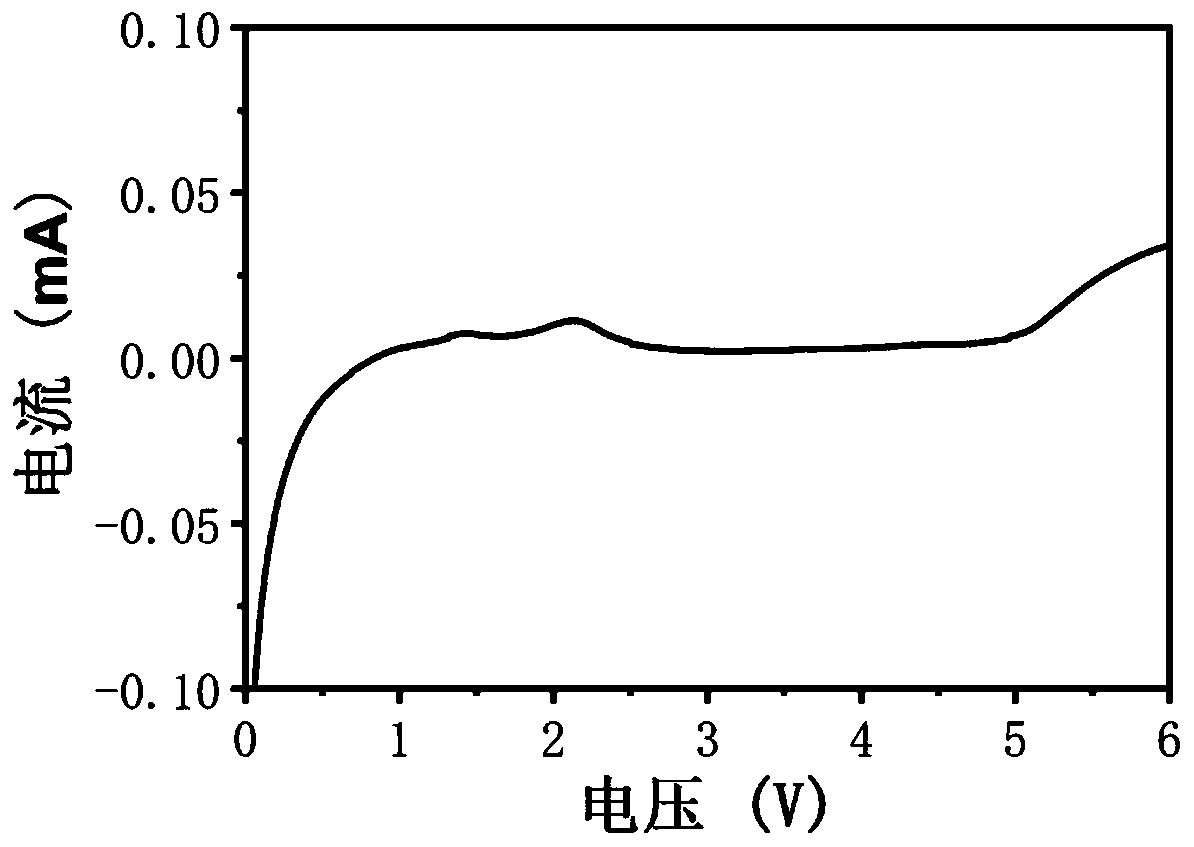
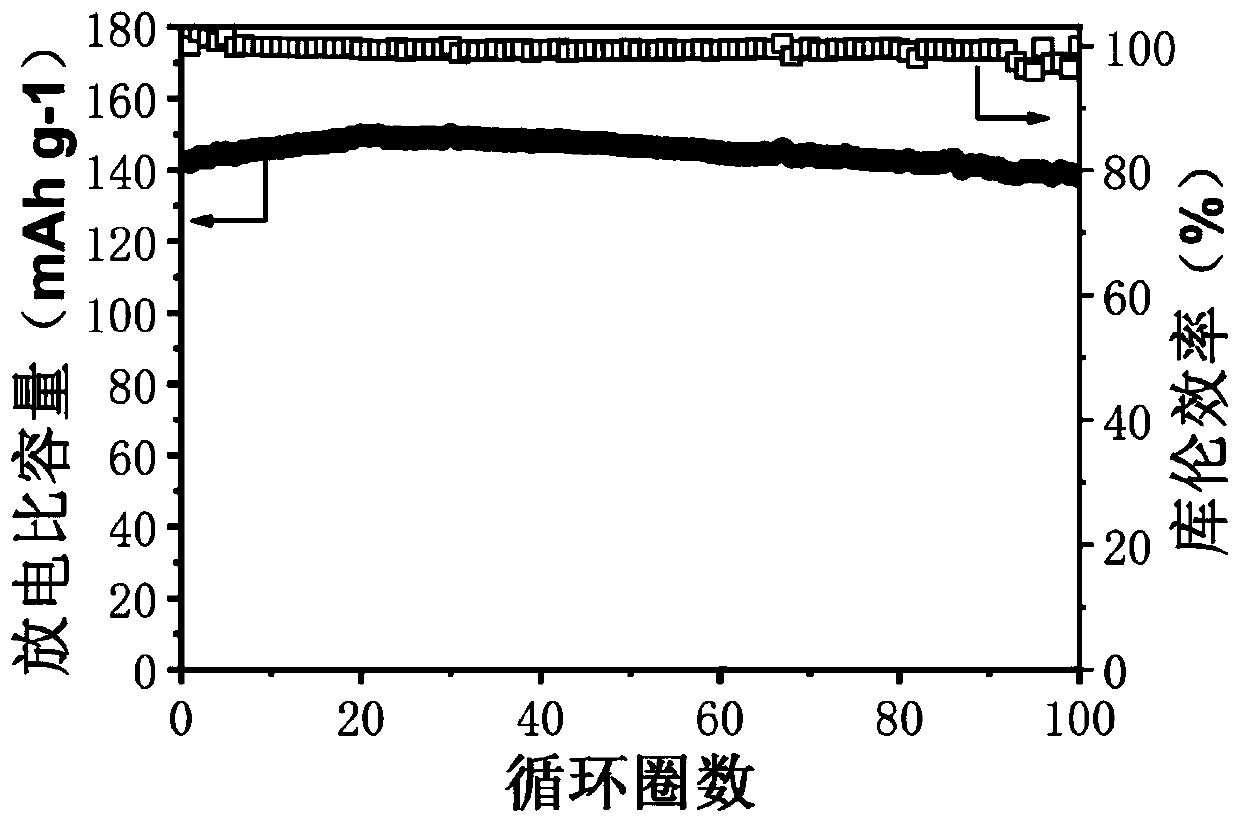
[0061] In this embodiment, a bifunctional self-healing polymer electrolyte includes a cross-linking agent with a disulfide bond, polyethylene glycol with a double bond, an initiator and a chain transfer reagent, wherein the cross-linking agent with a disulfide bond is It is obtained by reacting 2-isocyanoethyl acrylate with cystamine dihydrochloride, and the molar ratio of the two is 1:6; polyethylene glycol with double bonds is polyethylene glycol methyl ether methacrylate, polyethylene glycol The molecular weight of alcohol methyl ether methacrylate is 2000, and the molar ratio of it to the crosslinking agent with disulfide bond is 100:10. Lithium perchlorate is added to the above self-healing polymer, and the amount added is based on self-healing polymerization The molar ratio of the ethoxy segment in the compound to the Li ion is 25:1; the preparation method of the bifunctional self-healing polymer electrolyte in the present embodiment is as follows;
[0062] S1: React 5.5...
Embodiment 2
[0070] In this embodiment, a bifunctional self-healing polymer electrolyte includes a cross-linking agent with a disulfide bond, polyethylene glycol, an initiator and a chain transfer agent, wherein the cross-linking agent with a disulfide bond is methacrylic acid iso It is obtained by reacting cyanoethyl ester with 4,4'-diaminodiphenyl disulfide, the molar ratio of the two is 1:5; the polyethylene glycol with double bond is methoxypolyethylene glycol acrylate, methoxy The molecular weight of polyethylene glycol acrylate is 2000, and the molar ratio of it to the crosslinking agent with disulfide bonds is 100:15; lithium bistrifluoromethanesulfonimide is added to the above self-healing polymer, and the amount added is According to the molar ratio of the ethoxy segment in the self-healing polymer to the Li ion is 20:1; the preparation method of the bifunctional self-healing polymer electrolyte in this embodiment is as follows;
[0071] S1: React 3.1g of isocyanoethyl methacrylat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical conductivity | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com