Application of aspirin in preparation of platelet targeted drug delivery system
A technology of aspirin and platelets, which is applied in the field of medicinal chemistry, can solve problems such as difficult encapsulation of nanoparticles and drugs, platelet aggregation deformation, and thrombus formation, and achieve the effects of promoting release, preventing thrombus formation, and increasing output rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1: Aspirin inhibits platelet aggregation
[0030] In order to maintain the complete structural shape of platelets during in vitro treatment, avoid activation deformation, and achieve high platelet yield and high drug loading rate during drug loading, platelet activation must be strictly controlled. Select aspirin to inhibit platelet aggregation, and the preparation process is as follows:
[0031] 1. Wash the platelets and adjust the platelet concentration;
[0032] 2. Prepare aspirin solution so that the final concentration of aspirin solution in platelets is 1 ug / ml;
[0033] 3. Co-incubation of platelets and aspirin solution;
[0034] 4. Washing mixed solution;
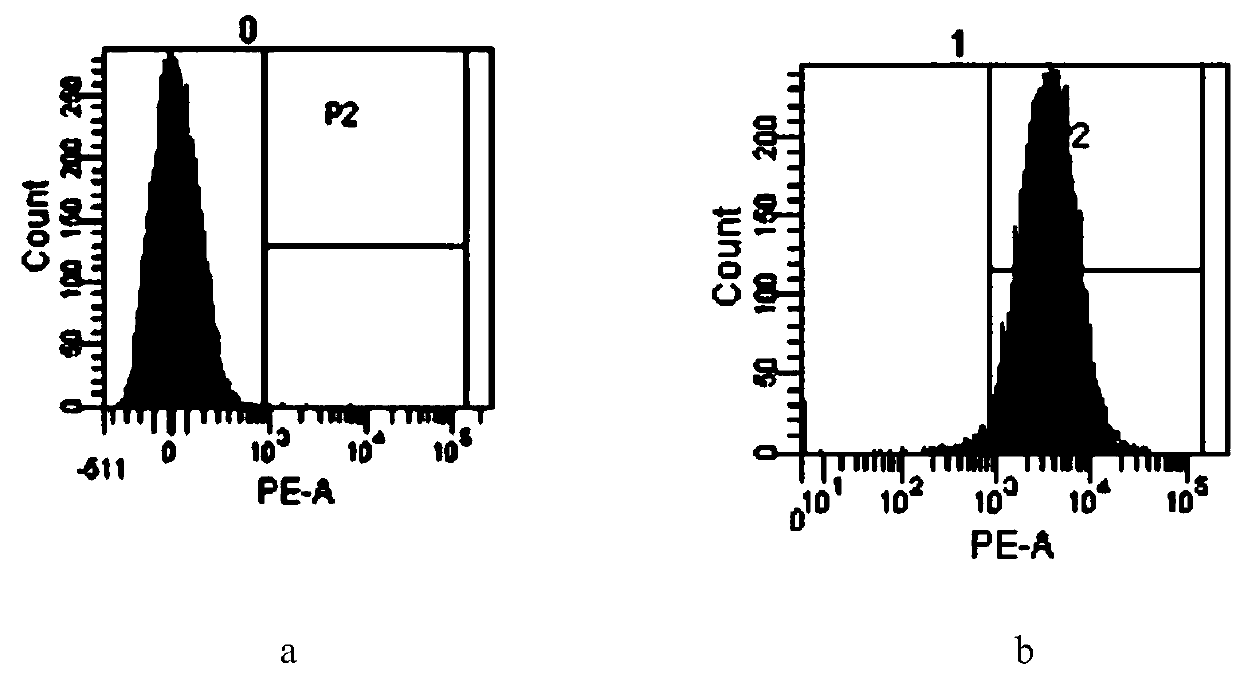
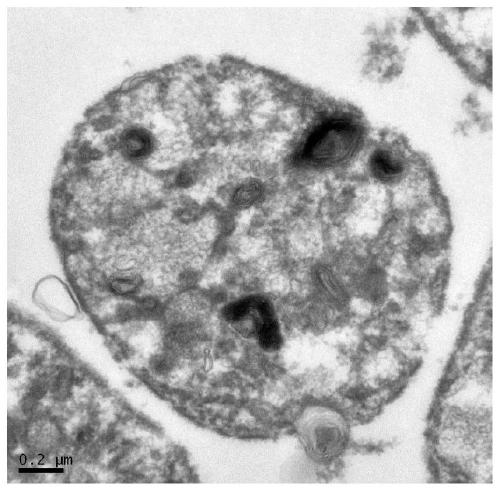
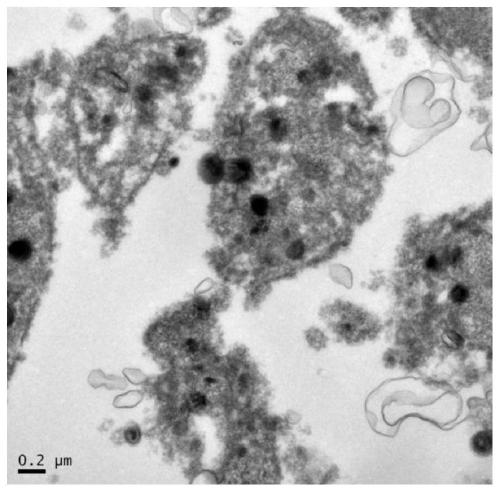
[0035] The relevant characterization after preparation, the flow diagram and electron microscope picture of the relevant structure are as follows figure 1 and figure 2 , 3 Shown:
[0036] From its flow graph ( figure 1 ) It can be seen that low-concentration aspirin is easier to enter platele...
Embodiment 2
[0038] Embodiment 2: Preparation and drug encapsulation of PLGA nanoparticles
[0039] PLGA is composed of random polymerization of two monomers - lactic acid and glycolic acid. It is a degradable functional polymer organic compound with good biocompatibility, non-toxicity, and good encapsulation and film-forming properties. Widely used in pharmaceuticals, medical engineering materials and modern industrial fields. Prepare PLGA nanoparticles, and encapsulate doxorubicin and new indocyanine green with PLGA nanoparticles to achieve drug stability.
[0040] making process:
[0041] 1. Weigh 80mg of PLGA, dissolve in 1ml of dichloromethane solution, and shake to fully dissolve;
[0042]2. Weigh 10mg of PVA, dissolve in 10ml of ultrapure water, soak overnight, and bathe in a water bath at 60°C for 4 hours;
[0043] 3. Weigh 1mg doxorubicin and new indocyanine green respectively and dissolve in 1ml ultrapure water;
[0044] 4. Place the PVA solution on a magnetic stirrer and sti...
Embodiment 3
[0045] Embodiment 3: Preparation of nano-platelets and encapsulation of PLGA nanoparticles
[0046] making process:
[0047] 1. Platelet preparation after aspirin inhibition. Ultrasonic crushing to obtain a mixed solution of nano-platelets;
[0048] 2. Ultrasonic dispersion and fusion of nanoplatelets and PLGA nanoparticles that have completed drug encapsulation to realize the encapsulation of nanoparticles by platelets;
[0049] 3. Transmission electron microscopy characterization.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com