Stable isotope labeled furaltadone metabolite and synthesis method thereof
A technology of stable isotope and synthesis method, which is applied in the field of stable isotope-labeled furotadone metabolites and their synthesis, can solve the problems of restricting the development of the veterinary drug residue detection industry, expensive isotope-labeled raw materials, difficult production process, etc., and achieves good economic The effect of high stability, high atomic utilization rate and high use value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0020] The synthetic method of the stable isotope-labeled furaltadone metabolite provided by the invention comprises the following steps:
[0021] S1: reacting stable isotope-labeled epichlorohydrin with morpholine to prepare stable isotope-labeled morpholino-based propylene oxide; the molecular structure of the stable isotope-labeled epichlorohydrin is:
[0022]
[0023] The molecular structure of the morpholino propylene oxide labeled with the stable isotope is:
[0024]
[0025] S2: react the stable isotope-labeled morpholino propylene oxide with hydrazine hydrate to obtain stable isotope-labeled 1-hydrazino 3-morpholine-2 propanol; the stable isotope-labeled 1-hydrazino 3- The molecular structure of morpholine-2 propanol is:
[0026]
[0027] S3: Under alkaline conditions, react the stable isotope-labeled 1-hydrazino 3-morpholine-2 propanol with a substituted formate to obtain a stable isotope-labeled furaltadone metabolite; the stable isotope The molecular stru...
Embodiment 1
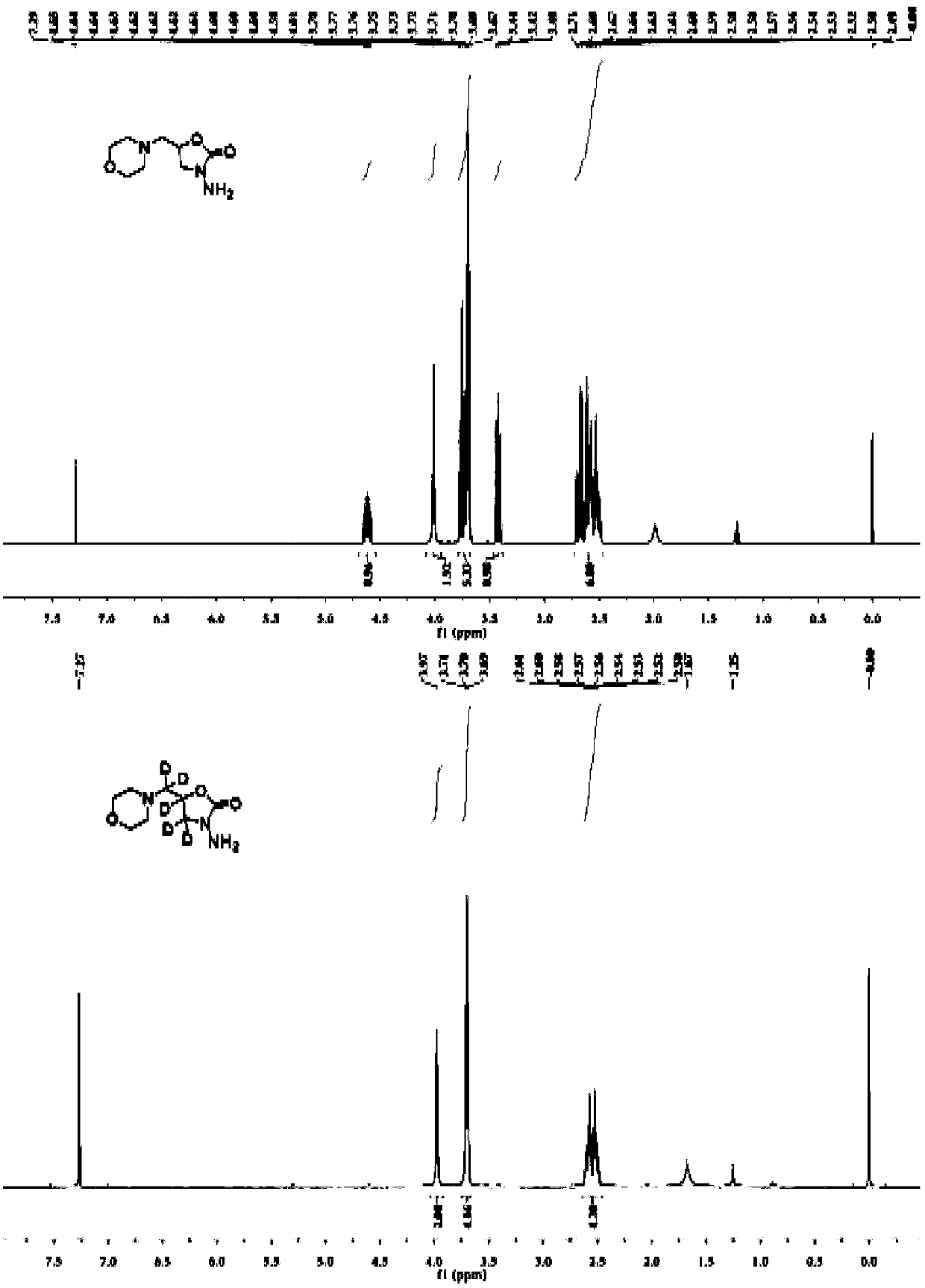
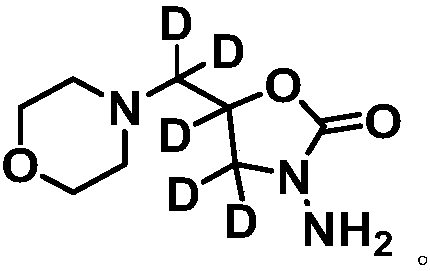
[0030] The molecular structure of the stable isotope-labeled furaltadone metabolite is as follows:
[0031]
[0032] Prepared by the following synthetic steps:
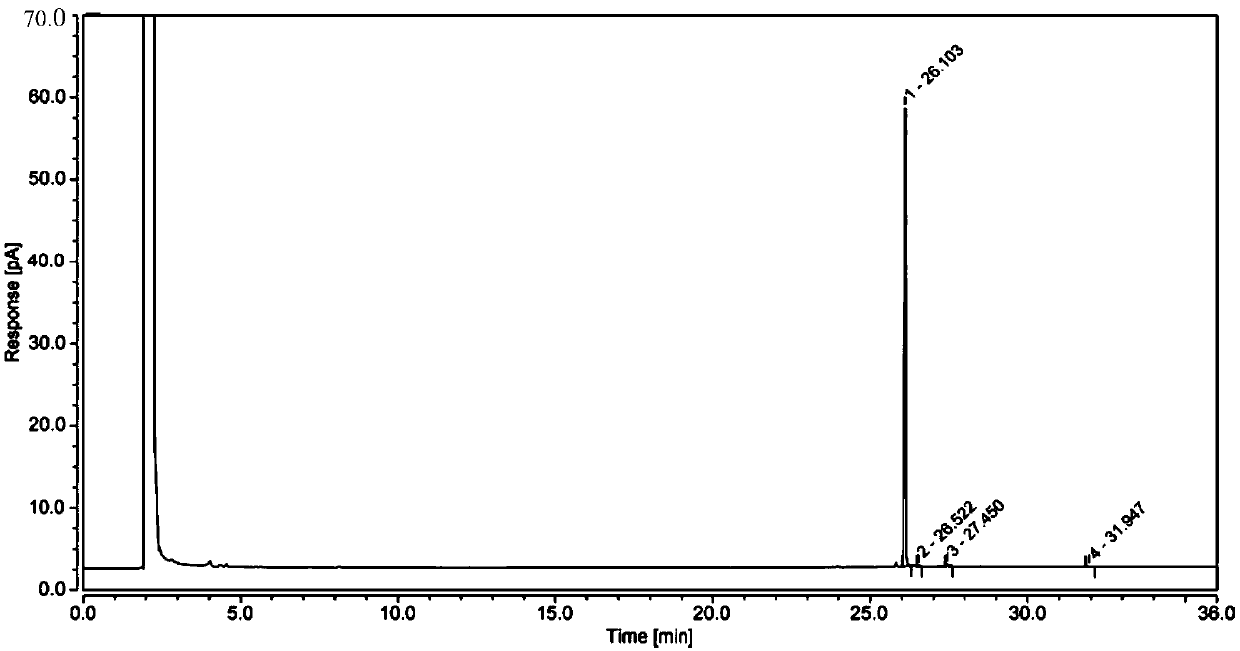
[0033] S1. Add 3.7g morpholine and 5.0g tert-butanol to the reaction vessel, place it in an ice-salt bath, add 4.0g epichlorohydrin-D5 dropwise through a constant pressure dropping funnel, and control the temperature during the dropping process at 0 ~27°C, the dropping time is 30~40min. After the dropping is completed, keep the reaction at 20~30°C for 18 hours. 25g 20wt% tetrahydrofuran solution of potassium tert-butoxide, the dropwise addition process controls the temperature at 0-15°C, after the dropwise addition is completed, the temperature is kept at 10-15°C for 2 hours, and column chromatography is used to obtain morpholino propylene oxide-D5 ;
[0034]S2. Add 5.0g morpholino propylene oxide-D5, 40mL ethanol, 6.5g 80% hydrazine hydrate to the reaction vessel, keep it warm at 80-90°C for 3 hours, and distill...
Embodiment 2
[0039] The synthesis process of stable isotope-labeled furaltadone metabolites is as follows:
[0040] S1. Add 4.0g morpholine and 6.0g tert-butanol to the reaction vessel, place it in an ice-salt bath, add 4.0g epichlorohydrin-D5 drop by drop through a constant pressure dropping funnel, and control the temperature during the dropping process at 0 ~27°C, the dropping time is 30~40min. After the dropwise addition is completed, keep the reaction at 20~30°C for 20 hours, then place the reaction vessel in an ice-salt bath, and add dropwise through a constant pressure dropping funnel. 30g of 20wt% tetrahydrofuran solution of potassium tert-butoxide, the dropwise addition process controls the temperature at 0-15°C, after the dropwise addition is completed, it is incubated at a temperature of 10-15°C for 2.5 hours, and column chromatography obtains morpholino propylene oxide- D5;
[0041] S2. Add 5.0g morpholino propylene oxide-D5, 50mL ethanol, 6.0g 85% hydrazine hydrate to the rea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com