Catalyst for biphenyl preparation as well as preparation method and application thereof
A technology of catalyst and biphenyl, which is applied in the field of catalysts for the preparation of biphenyl and its preparation, can solve the problems of limited application, difficulty in modifying the neutral skeleton structure, and low yield, and achieve high catalytic efficiency, high utilization rate of precious metals, and preparation The effect of simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
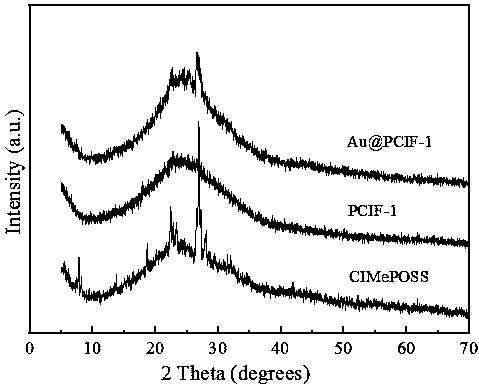
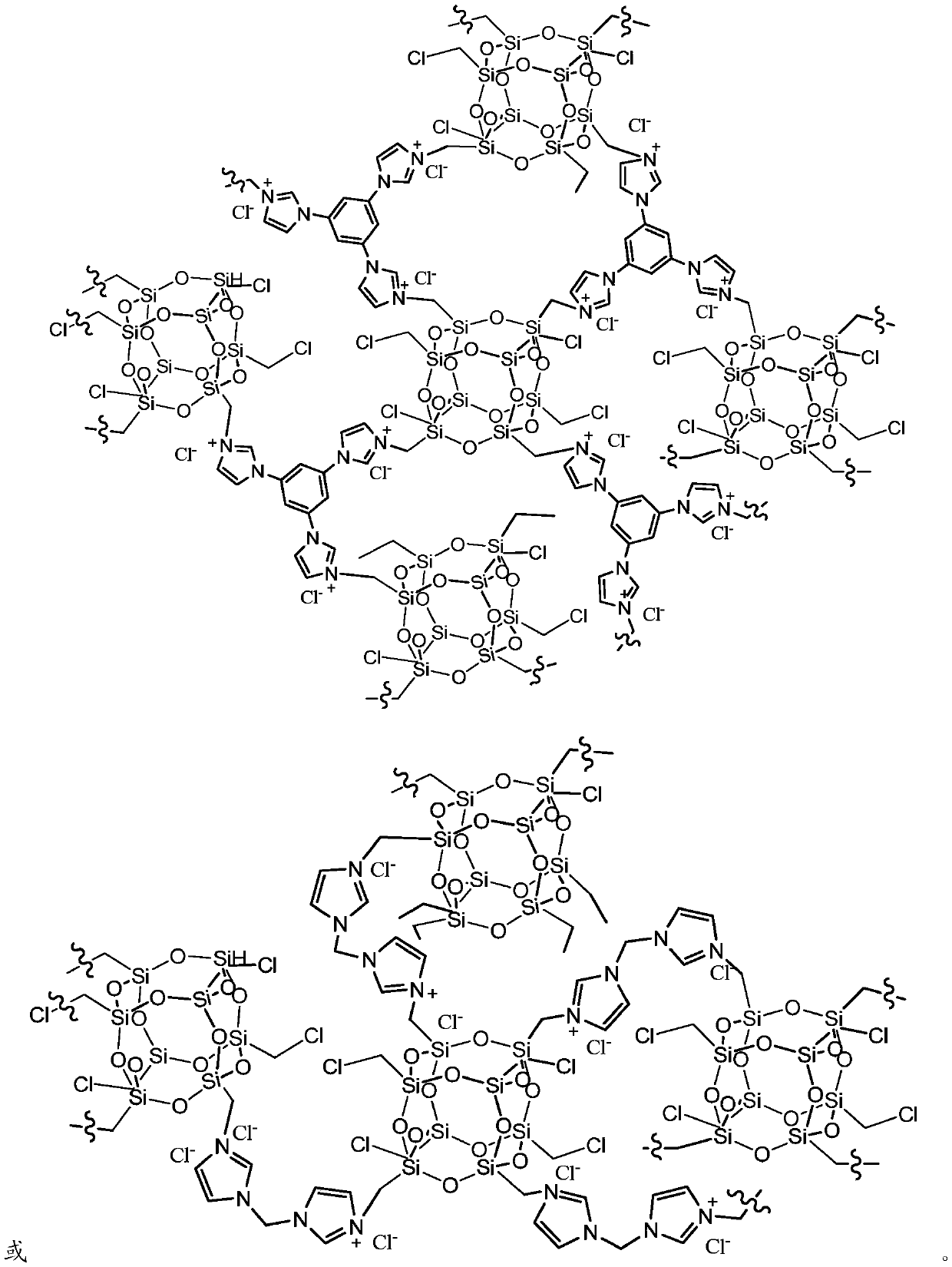
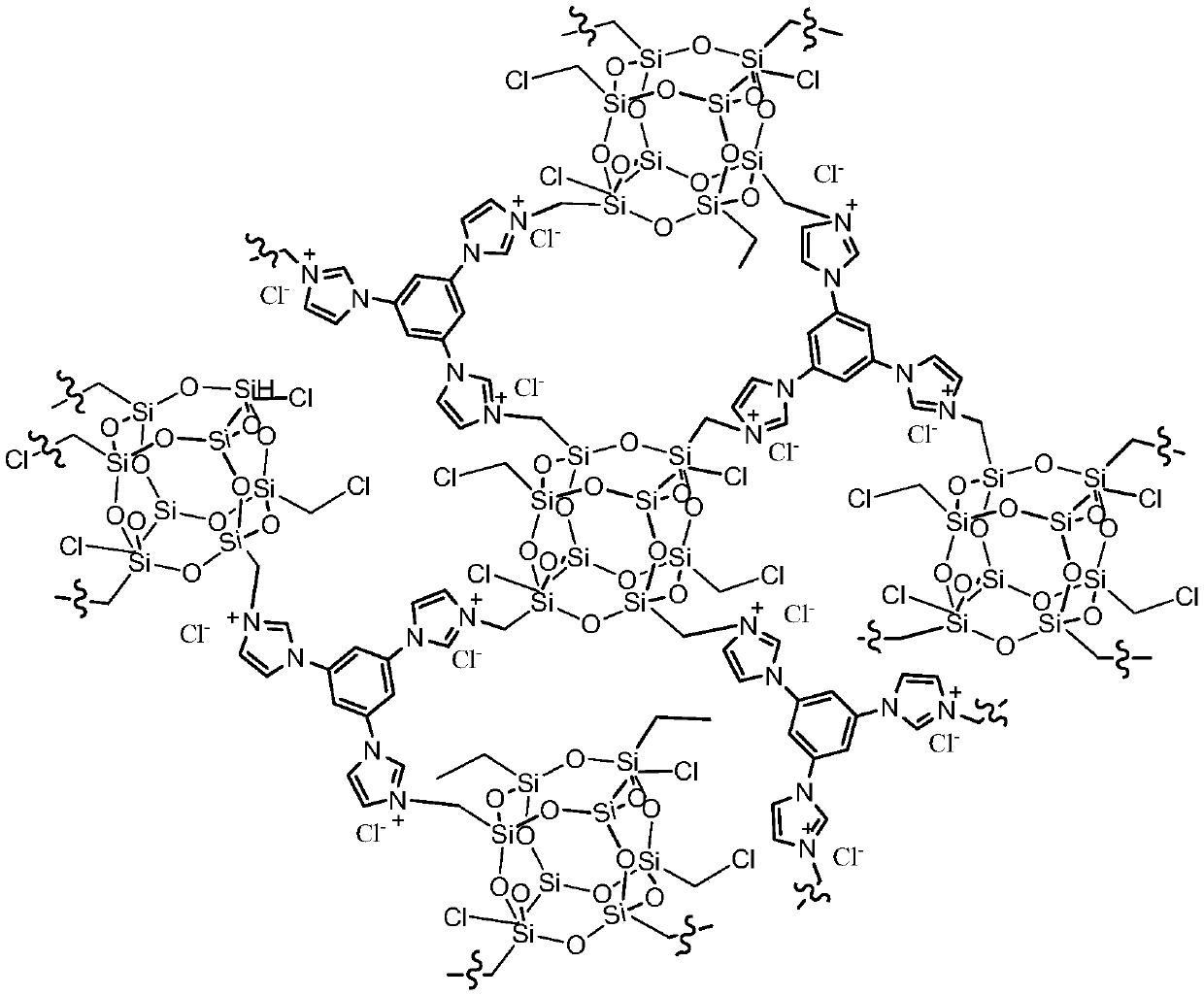
Image
Examples
Embodiment 1
[0031] Add 200 mL of anhydrous methanol and 6 mL of concentrated hydrochloric acid into a 250 mL round bottom flask, and slowly add 20 g of chloromethyltriethoxysilane while stirring at room temperature. The above solution was heated and sealed in a water bath at 40°C for two weeks. After the reaction, the solvent was removed by rotary evaporation and dried in vacuo to finally obtain a white crystal product, namely ClMePOSS, with a yield of 90%.
[0032] 0.6 g of ClMePOSS and 0.4 g of trimidazolylbenzene were dissolved in 10 mL of THF, and then the solution was placed in a Teflon-lined stainless steel reactor and reacted statically in an oven at 100 °C for 48 h. After the reaction, the resulting gel-like monolithic solid was cut into small pieces and stirred in an ethanol solvent for 2 hours, and then the dispersed ionic colloidal suspension solution was suction-filtered, thoroughly washed with ethanol, THF, and water, and vacuum-dried. Finally, a light brown solid product is...
Embodiment 2
[0038] Add 100 mL of anhydrous methanol and 4 mL of concentrated hydrochloric acid into a 250 mL round bottom flask, and slowly add 10 g of chloromethyltriethoxysilane under stirring at room temperature. The above solution was heated and sealed in a water bath at 40°C for two weeks. After the reaction, the solvent was removed by rotary evaporation and dried in vacuo to finally obtain a white crystal product, namely ClMePOSS, with a yield of 90%.
[0039] 0.3 g of ClMePOSS and 0.4 g of 1,2-diimidazolethane were dissolved in 10 mL of THF, and then the solution was placed in a Teflon-lined stainless steel reactor and reacted statically in an oven at 100 °C for 48 h. After the reaction, the resulting gel-like monolithic solid was cut into small pieces and stirred in an ethanol solvent for 2 hours, and then the well-dispersed ionic colloidal suspension solution was suction-filtered, thoroughly washed with ethanol, THF, and water, and vacuum-dried. , and finally obtain a light brow...
Embodiment 3
[0045] Put 1.5mmol benzene, 0.02gAu@PCIF-1, 5mL acetic acid, and 0.02g trifluoroacetic acid in a closed high-pressure reactor and mix evenly. Oxygen is introduced into the reactor, and the pressure of the reaction system in the reactor is 8 atm. Maintain the temperature of the reactor At 120° C., oxidative coupling reaction was carried out for 10 h to obtain biphenyl, the yield of biphenyl was 3.4%, the conversion rate of benzene was 3.5%, and the selectivity of biphenyl was 95.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com