High-biosafety heart stent and manufacturing method thereof
A biosafety and heart stent technology, which is applied in pharmaceutical formulation, drug delivery, medical science, etc., can solve problems affecting the blood compatibility of the stent, insufficient biosafety, and insufficient strength of the stent, so as to improve blood compatibility , Improve the strength and hydrophilicity, and the effect of excellent degradation performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
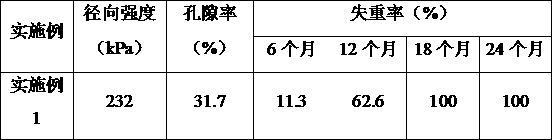
Embodiment 1
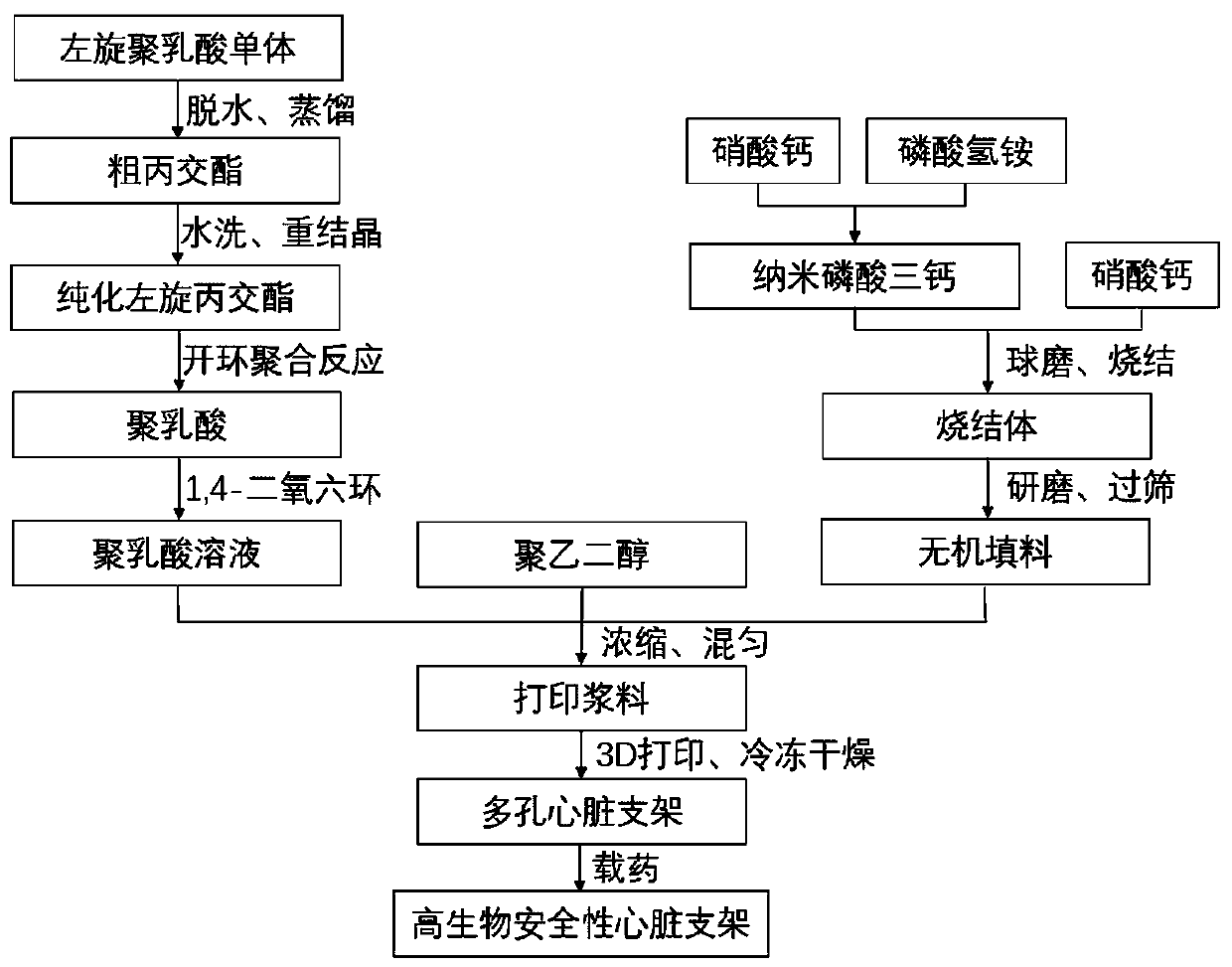
[0054] This embodiment provides a method for manufacturing a high biosafety heart stent, which includes the following steps:
[0055] S1. Polylactic acid is prepared by a lactide ring-opening polymerization method, and the polylactic acid is dissolved in 1,4-dioxane at a mass-volume ratio of 1g:10mL, and fully stirred before use;
[0056] The preparation of polylactic acid by the lactide ring-opening polymerization method includes the following steps:
[0057] S11. Put the L-lactic acid monomer in a round-bottom flask, heat and reduce pressure for dehydration at 170℃, 5kPa for 5h, then add 1wt% of stannous octoate as a catalyst, and carry out propylene at 200℃, 0.5kPa Distillation of lactide and condensation to obtain crude lactide;
[0058] S12. The crude lactide obtained in step S11 is washed and recrystallized in sequence to obtain purified L-lactide; the washing process includes placing the crude lactide in deionized water, rapidly stirring and then performing suction filtration ...
Embodiment 2~5 and comparative example 1
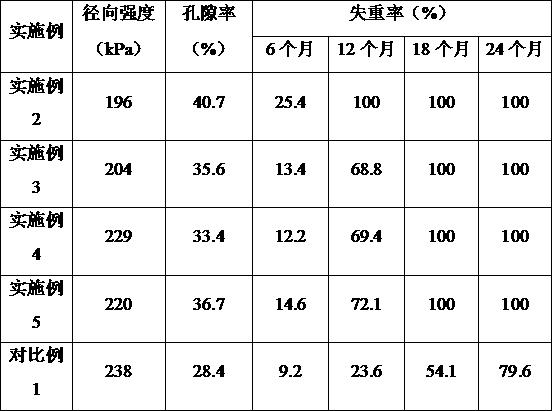
[0069] Examples 2 to 5 and Comparative Example 1 respectively provide a method for manufacturing a high biosafety heart stent. Compared with Example 1, the difference is that Examples 2 to 5 change the time of the ring-opening polymerization reaction in step S13. , Thereby obtaining polylactic acid with different molecular weights; Comparative Example 1 did not prepare polylactic acid through step S1, but directly purchased commercially available polymer L-polylactic acid with a molecular weight of 900,000. The ring-opening polymerization reaction time corresponding to each example and the molecular weight of polylactic acid in each example and comparative example are shown in Table 2.
[0070] Table 2 The ring-opening polymerization reaction time of Examples 2 to 5 and Comparative Example 1 and the molecular weight of polylactic acid
[0071] Example Ring-opening polymerization reaction time (h) Polylactic acid molecular weight Example 212 400000 Example 318 600000 Example...
Embodiment 6~9 and comparative example 2
[0079] Examples 6 to 9 and Comparative Example 2 respectively provide a method for manufacturing a high biosafety cardiac stent. Compared with Example 1, the difference is that Examples 6 to 9 change the nano-tricalcium phosphate and nano-tricalcium phosphate in step S3. The mass ratio of magnesium oxide or the sintering temperature and sintering time of the sintering process; in Comparative Example 2, nano-tricalcium phosphate was directly used as the inorganic filler, without the addition of magnesium oxide and the sintering process. The raw material mass ratio and sintering parameters corresponding to each embodiment and comparative example are shown in Table 4.
[0080] Table 4 The raw material mass ratio and sintering parameters of step S3 in Examples 6-9 and Comparative Example 2
[0081]
[0082] The radial strength, porosity and degradation performance of the high biosafety cardiac stents prepared in Examples 6-9 and Comparative Example 2 were tested, and the results are sh...
PUM
| Property | Measurement | Unit |
|---|---|---|
| strength | aaaaa | aaaaa |
| strength | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com