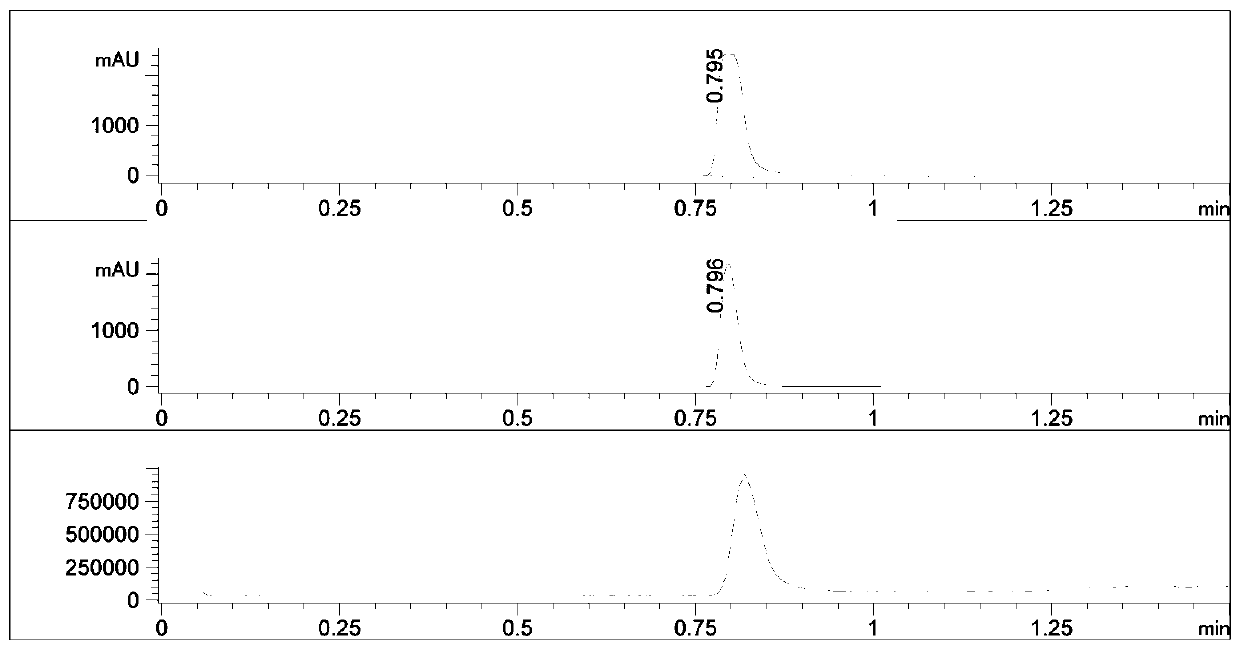
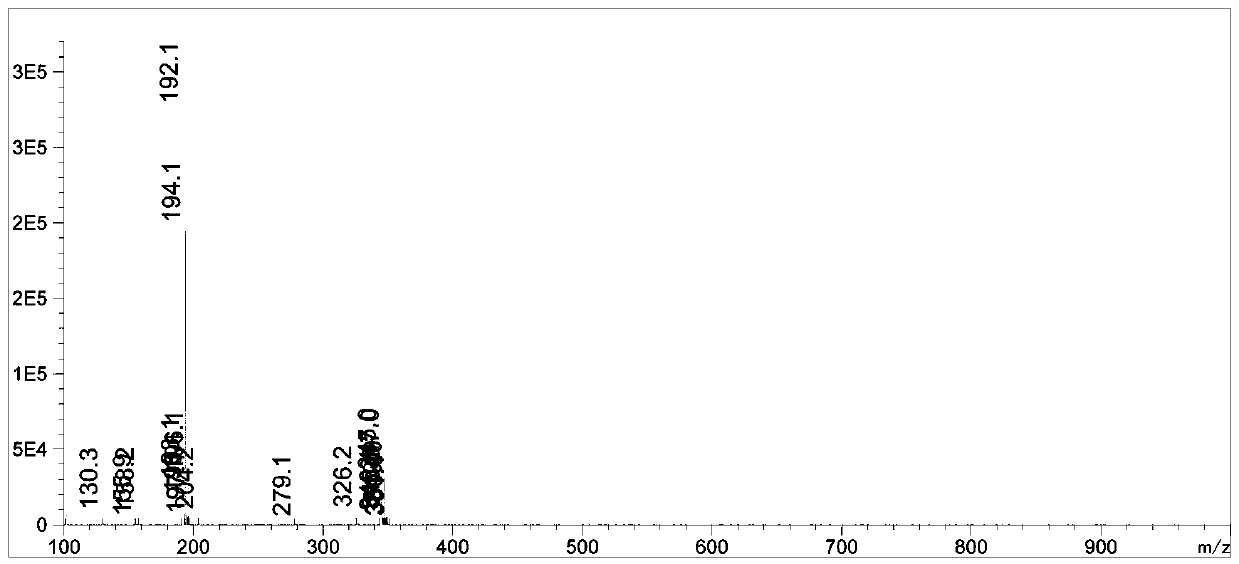
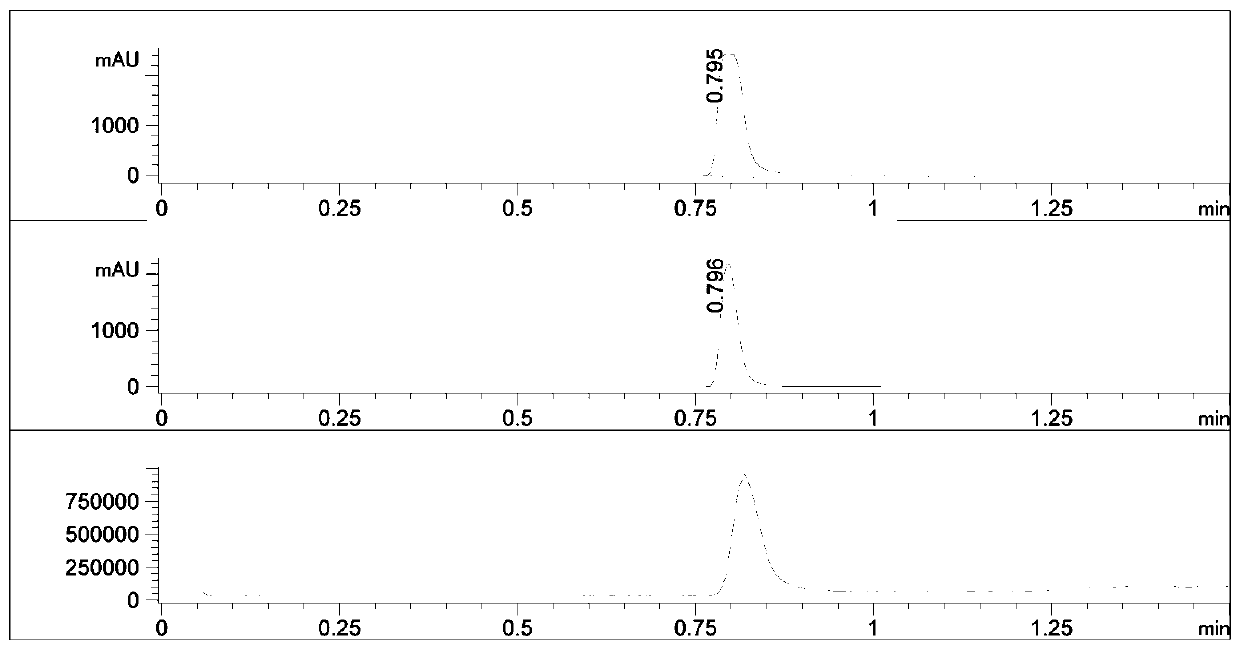
Synthetic method of bosutinib intermediate
A technology of bosutinib and a synthesis method, applied in the field of chemistry, can solve the problems of increased environmental protection pressure, difficult recycling and application, and high boiling point difference, and achieves the effects of convenient post-processing and purification, good reaction selectivity, and easy removal.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] The synthesis method of the bosutinib intermediate, the bosutinib intermediate is 2,4-dichloro-5-methoxyaniline, add 545g of formic acid to a 3000mL three-necked flask, start stirring, and add m-aminophenol in batches 109g (1.0mol), 1.82g (10mmol) of vanadium pentoxide, heated to 100°C, TLC monitored the completion of the reaction (PE:EA=1:1), cooled to room temperature, and reacted with chlorine gas in about 3h, the color Turned into light yellow, spot plate to monitor the completion of the reaction (PE: EA = 1: 1). After the reaction is finished, filter, and the filtrate recovers formic acid for recycling. The intermediate was directly carried out to the next reaction without purification. Add 1500mL of dimethylformamide and 207.3g (1.5mol) of potassium carbonate to the reaction flask filled with 2,4-dichloro-5-carboxamidophenol that recovered the complete formic acid, stir at 40°C for 30min, then add dropwise for about 1h 189.2 g (1.5 mol) of dimethyl sulfate was h...
Embodiment 2
[0042] Add 1417g of formic acid to a 3000mL three-necked flask, start stirring, add 109g (1.0mol) of m-aminophenol and 16.2g (70mmol) of tungsten trioxide in batches, heat to 50°C, and monitor the completion of the reaction by TLC (PE:EA=1: 1), then cooled to 20°C, cooled to room temperature, and added dropwise 337.4g (2.5mol) of sulfuryl chloride. After the dropwise addition, the color gradually changed to light yellow, and the reaction was completed by spotting the plate to monitor (PE:EA=1:1). After the reaction is finished, filter, and the filtrate recovers formic acid for recycling. Add an appropriate amount of dichloroethane to the reaction flask where the formic acid has been recovered, dissolve it, wash it with water until neutral, and concentrate to obtain the crude product of 2,4-dichloro-5-carboxamidophenol, which is directly carried out to the next step reaction without purification. Transfer all the obtained crude 2,4-dichloro-5-carboxamidophenol into a 3000mL aut...
Embodiment 3
[0045]Into a 3000mL three-neck flask, add 1090g of formic acid, start stirring, add 109g (1.0mol) of m-aminophenol, 9.1g (50mmol) of vanadium pentoxide in batches, heat to 70°C, and monitor the completion of the reaction by TLC (PE:EA=1 : 1), then cooled to 40°C, added 175.3g (3.0mol) of sodium chloride in batches, added dropwise 510.2g (4.5mol) of 30% hydrogen peroxide in 30 minutes, and monitored the reaction by TLC (PE:EA=1:1) , After the reaction is finished, filter, and the filtrate recovers formic acid for recycling. The intermediate was directly carried out to the next reaction without purification. Add 1500mL of propanol, 165.9g (1.2mol) of potassium carbonate to the 3000mL reaction flask filled with 2,4-dichloro-5-carboxamidophenol that recovered complete formic acid, stir at 40°C for 30min, add disulfuric acid dropwise for about 1h 151.4 g (1.2 mol) of methyl ester was heated up to 50° C., and stirring was continued for 1 h to obtain a white suspension. The reactio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com