A group of epothilone b glucoside compounds and their enzymatic preparation and application
A technology of epothilone and glucoside, which is applied in the application field of epothilone B glucose monosaccharide, epothilone glycoside compound and its preparation and application, and medicine, and can solve the difficulty of organic chemical synthesis method, Problems such as high synthesis cost and many by-products have achieved good social benefits and economic value, increased application value, and novel effects on targets
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
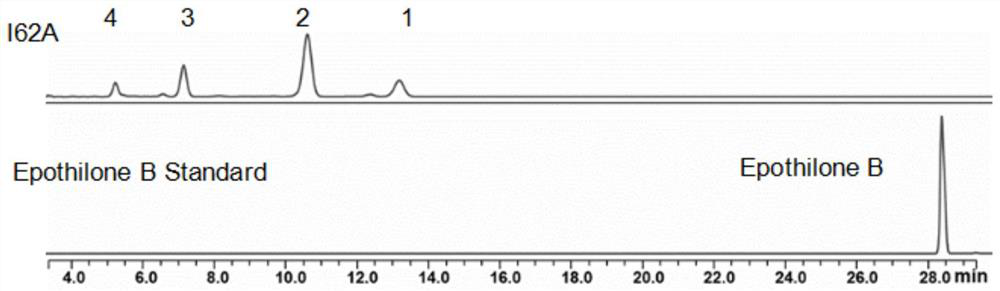
[0030] Example 1 Acquisition of Epothilone Glycosyltransferase BsGT-1 Recombinant Plasmid, Construction of I62A Mutant Recombinant Plasmid, Expression of I62A Mutant Protein, and Preparation of Epothilone B Glucoside
[0031] The inventor screened and determined the glycosyltransferase BsGT-1 (CUB50191) capable of efficiently glycosylation of epothilone B, and its protein sequence has been published. Obtaining the epothilone glycosyltransferase BsGT-1 recombinant plasmid and expressing the method for obtaining the glycosyltransferase BsGT-1 is:
[0032] The gene fragment encoding glycosyltransferase BsGT-1 was obtained by extracting the genomic DNA of B. subtilis JRS11, and double restriction primers were designed (F-BamHI: CGCGGATCCATGAAAAAGTACCATATTTCGAT; R-SalI: ACGCGTCGACTTACTGCGGGACAGCGGATTTTT). Max Super-Fidelity DNA Polymerase high-fidelity polymerase for polymerase chain reaction (PCR), reaction system: B.subtilis JRS11 genomic DNA 0.5μl; 2×PhantaMax Buffer 25μl; dNT...
Embodiment 2
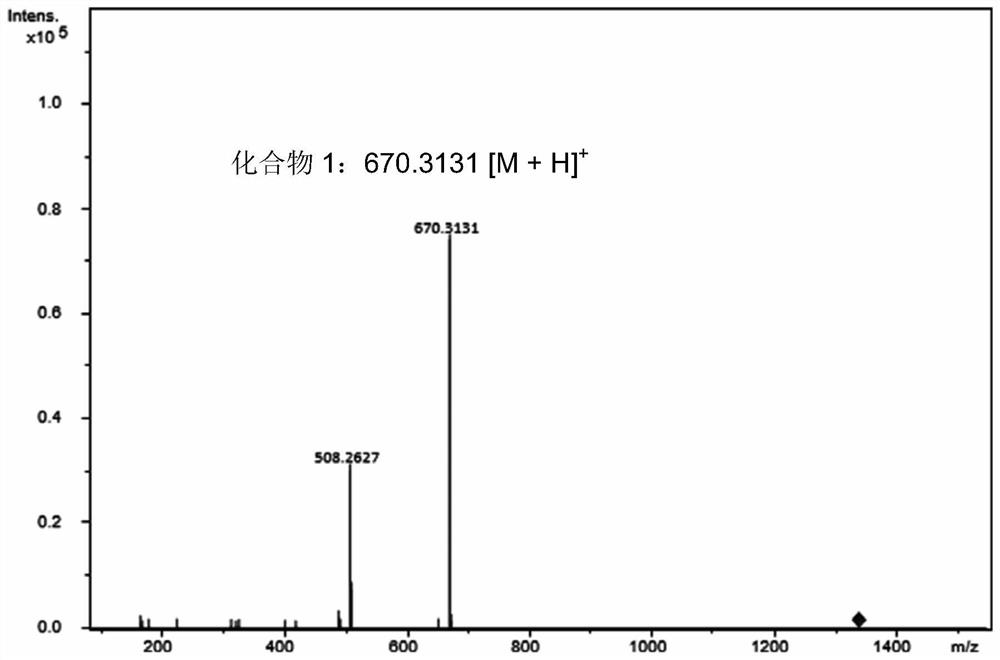
[0042] The structure identification of embodiment 2 compound 1
[0043] in accordance with figure 2 , UHPLC-ESI-Q-TOF high-resolution mass spectrometry gives the quasi-molecular ion peak of compound 1 [M+H] + It is m / z670.3131, thus confirming that 1 is the monoglucoside of epothilone B. Simultaneously, the monosaccharide can also be obtained from the H NMR spectrum of compound 1 ( 1 H NMR) and carbon spectrum ( 13 C NMR) to be confirmed. In the HMBC spectrum, H-7 is related to C-1' and H-1' is related to C-7, thus confirming that the glucosyl group is connected to the 7-hydroxyl group of the epothilone B macrolide skeleton. The large coupling constant between the anomeric protons H-1' and H-2' on the sugar (J 1',2' =7.8Hz) and the chemical shift value of 4.46ppm for the high field of the anomeric proton, revealing that the glycosyl donor and the epothilone B acceptor are linked by a β-D glucosidic bond. Therefore, compound 1 is EpothiloneB 7-O-β-Dglucoside, and its spe...
Embodiment 3
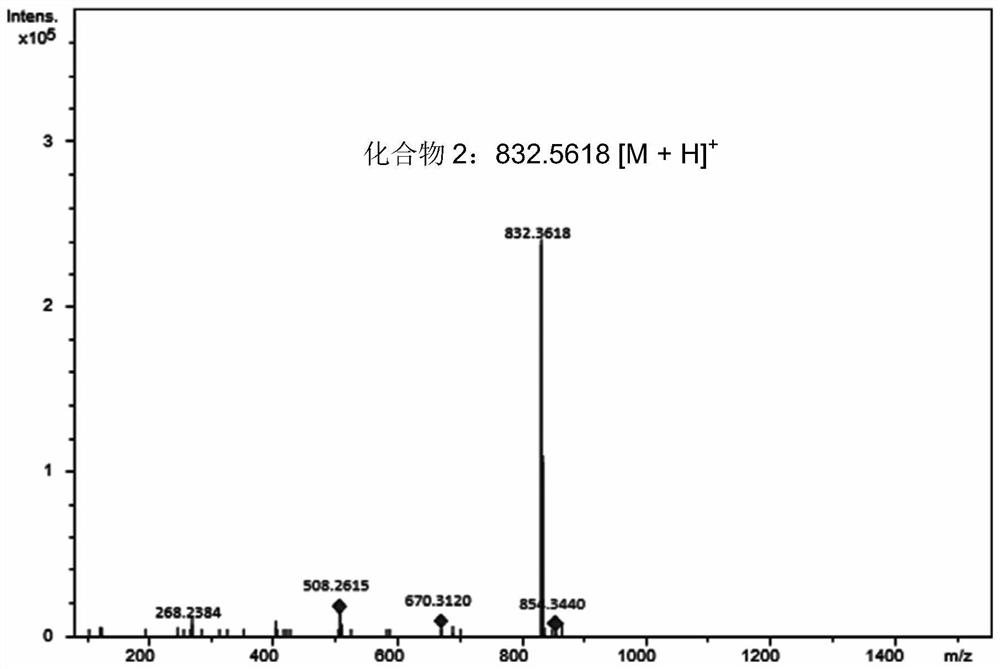
[0048] The structure identification of embodiment 3 compound 2
[0049] in accordance with image 3 , the high-resolution mass spectrum gives the quasi-molecular ion peak of compound 2 [M+H] + It is m / z832.5618, which is predicted to be the diglucoside of epothilone B. The secondary mass spectrum of compound 2 gives fragment ion peaks m / z 670.3120 and 508.2615, and proton nuclear magnetic resonance spectrum ( 1 H NMR) and carbon spectrum ( 13 C NMR) provides obvious signals of two sugars, further verifying that 2 is the diglucoside of epothilone B. Similarly, HMBC and sugar terminal proton coupling constant analysis (J 1',2' =7.8Hz), it was determined that the disaccharide group was connected to epothilone B through a 7-O-β-D glucosidic bond. As for the β-1,3-glucosidic bond connection mode inside the disaccharide, it is mainly determined by two-dimensional nuclear magnetic resonance experiments, including HSQC, HMBC, COZY and NOESY. Specifically, the terminal group prot...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com