In-vitro expression method for pear PMEI (pectin mthylesterase inhibitor) protein, and application of pear PMEI
An in vitro expression and protein technology, applied in the field of bioengineering, can solve the problems of high cost of PMEI and difficulty of PMEI protein
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Example 1: Construction and Identification of PMEI-pCold-TF Recombinant Plasmid
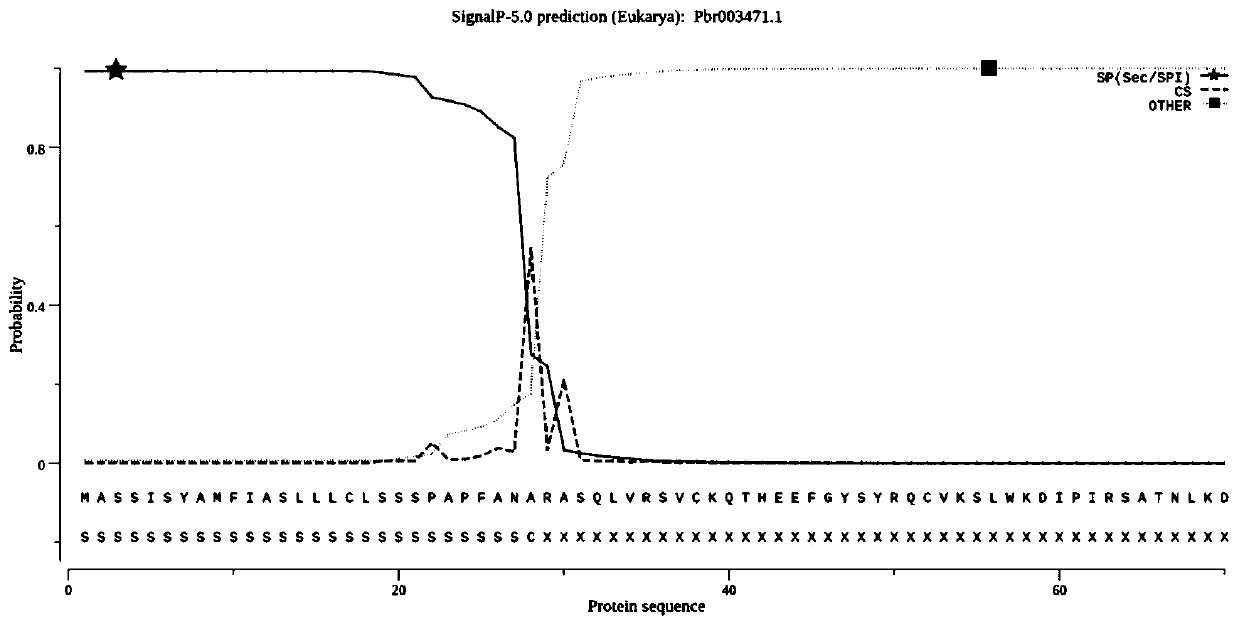
[0065] Fresh Dangshansu pear pollen was taken, and total pollen RNA was extracted with a plant total RNA extraction kit; pollen total RNA was used as a template, and pollen cDNA was obtained by reverse transcription with a reverse transcription kit; figure 1 As shown, the signal peptide sequence of the pear PMEI gene was analyzed and removed, primers were designed, and the homology arms of the vector pCold-TF and the restriction sites of Xba I and Xho I were added to both ends of the primers, and the pollen cDNA was used as a template. PCR amplification of PMEI gene, PCR reaction system: ddH 2 O 19 μl, each 2.5 μl of upstream and downstream primers, 1 μl of cDNA, 2×Super Pfx MasterMix 25 μl; the PCR reaction conditions were: 98°C pre-denaturation for 3 minutes, followed by 98°C for 10 seconds, 60°C for 30 seconds, 72°C for 1 minute, a total of 30 cycles, and finally 72°C ℃ extension 10mi...
Embodiment 2
[0067] Embodiment 2: the expression of pear PMEI gene in escherichia coli
[0068] 1. Obtain a recombinant expression strain expressing pear PMEI
[0069] The monoclonal strain with correct sequencing was inoculated into 4 ml LB liquid medium (containing 100 μg / ml ampicillin), and cultured with shaking at 37° C. and 220 rpm overnight. The next day, the recombinant plasmid PMEI-pCold-TF was extracted according to the instructions of the QuickPure Plasmid Mini kit of Kangwei Century Company. Transform 1ng of the recombinant plasmid PMEI-pCold-TF into Escherichia coli strain Rosetta (DE3) by heat shock method, spread on LB plates (containing 100 μg / ml ampicillin) to select recombinants, and culture overnight at 37°C to obtain the expression pear Genetically engineered bacteria of PMEI. Get the single bacterium colony of the obtained genetically engineered bacteria and streak culture on the LB plate (containing 100 μg / ml ampicillin), inoculate a small amount of streaked culture ...
Embodiment 3
[0072] Embodiment 3: Separation, purification and identification of recombinant pear PMEI protein
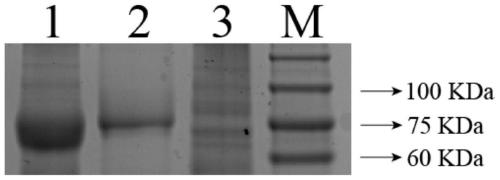
[0073] The above-mentioned induced expression for 24 hours and the correct remaining recombinant expression bacterial liquid detected by SDS-PAGE electrophoresis were centrifuged at 4°C and 10000rpm for 10 minutes, the supernatant was discarded and the bacterial precipitate was collected, and 20ml of lysate (140mM sodium chloride) was added to the precipitate. , 2.7mM potassium chloride, 10mM disodium hydrogen phosphate, 1.8mM potassium dihydrogen phosphate, 50×EDTA-free protease inhibitorcocktail III (Merck Millipore, Germany), pH 7.3) to resuspend the precipitate; (Ultrasonic power is 240w, the conditions are 3s on, 7s off, 27 minutes in total) After clarification, centrifuge at 4°C and 10,000rpm for 10 minutes, collect the supernatant, and filter the supernatant through a 0.45μm filter membrane to remove impurities. Libo's Ni-NTA agarose affinity chromatography filler was use...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com