A kind of preparation method of composite cathode material for fuel cell
A composite cathode, fuel cell technology, applied in battery electrodes, preparation/processing of rare earth metal compounds, nanotechnology for materials and surface science, etc. It can reduce the probability of cathode agglomeration, improve the catalytic efficiency, and the preparation process is simple.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0026] Preparation of LSCF powder:
[0027] (1) 5.1g La (NO 3 ) 3 ·6H 2 O, 1.7g Sr (NO 3 ) 2 , 1.2g Co(NO 3 ) 2 ·6H 2 O, 6.5g Fe(NO 3 ) 3 ·9H 2 O, 11.5g of citric acid, and 11.7g of EDTA powder were added to the beaker, mixed with 500mL of deionized water, and then slowly poured into 24mL of 25% aqueous ammonia solution, and kept stirring to fully dissolve, and pH=5 was measured at this time;
[0028] (2) The stirred colored solution was heated to 300°C again. After the solution was completely transformed into a gel, the solution was put into an oven for drying, and then calcined at 950°C for 5 hours to obtain pure LSCF cathode powder.
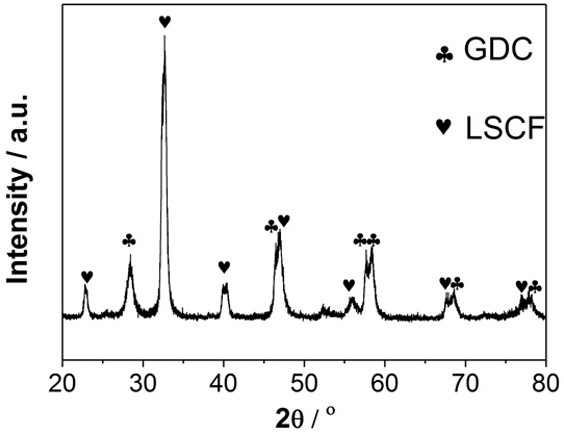
[0029] figure 1 is the SEM surface topography of the prepared pure LSCF cathode powder. As shown in the figure, the particle size is uneven, the agglomeration phenomenon is serious, and the dispersion is uneven.
Embodiment 1
[0030] Example 1 Preparation of LSCF / GDC composite powder
[0031] (1) Weigh 0.02g (4.6×10 -5 mol) Gd (NO 3 ) 3 ·6H 2 O, 0.22g (4.8×10 -4 mol) Ce (NO 3 ) 3 ·6H 2 O, 0.33g (1.7×10 -3 mol) citric acid, 0.33g (1.1×10 -3 mol) EDTA in a beaker, pour 100 mL of deionized water, slowly pour 0.7 mL of 25% ammonia solution, and keep stirring at room temperature to fully dissolve it, and the pH of the solution is detected to be 6;
[0032] (2) Heating and stirring the obtained pale yellow solution at 180°C, adding a certain amount of LSCF cathode powder when the residual water reaches 50 mL, then keeping the temperature unchanged, stirring and mixing to obtain a black bulky cathode precursor gel;
[0033] (3) Put the obtained precursor gel in a 180°C oven to dry for 10 hours, then take out the bulk precursor and grind it into powder, put it into a crucible, and calcine it in a high-temperature furnace at 750°C for 2 hours, and then take it out and grind it. Fine, 10wt% GDC-coat...
Embodiment 2
[0035] Example 2 Preparation of LSCF / GDC composite powder
[0036] (1) Weigh 0.04g (9.2×10 -5 mol) Gd (NO 3 ) 3 ·6H 2 O, 0.44g (9.6×10 -4 mol) Ce (NO 3 ) 3 ·6H 2 O, 0.66g (3.4×10 -3 mol) citric acid, 0.68g (2.2×10 -3 mol) EDTA in a beaker, pour 200 mL of deionized water, slowly pour 0.14 mL of ammonia aqueous solution (concentration of 25%), and keep stirring at room temperature to fully dissolve it. The pH value of the detected solution is 6;
[0037] (2) Heating and stirring the obtained pale yellow solution at 180°C, adding a certain amount of LSCF cathode powder when the residual water reaches 50 mL, then keeping the temperature unchanged, stirring and mixing to obtain a black bulky cathode precursor gel;
[0038] (3) Put the obtained precursor gel in a 180°C oven to dry for 10 hours, then take out the bulk precursor and grind it into powder, put it into a crucible and calcine it in a high temperature furnace at 750°C for 2 hours, then take it out and grind it fin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com