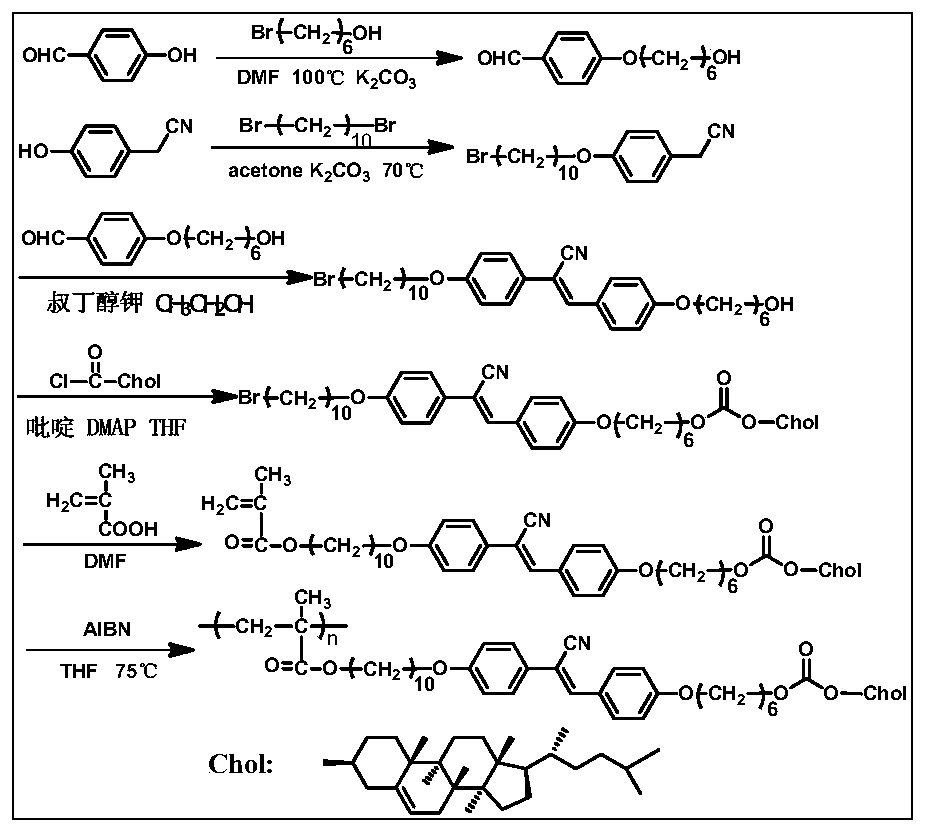
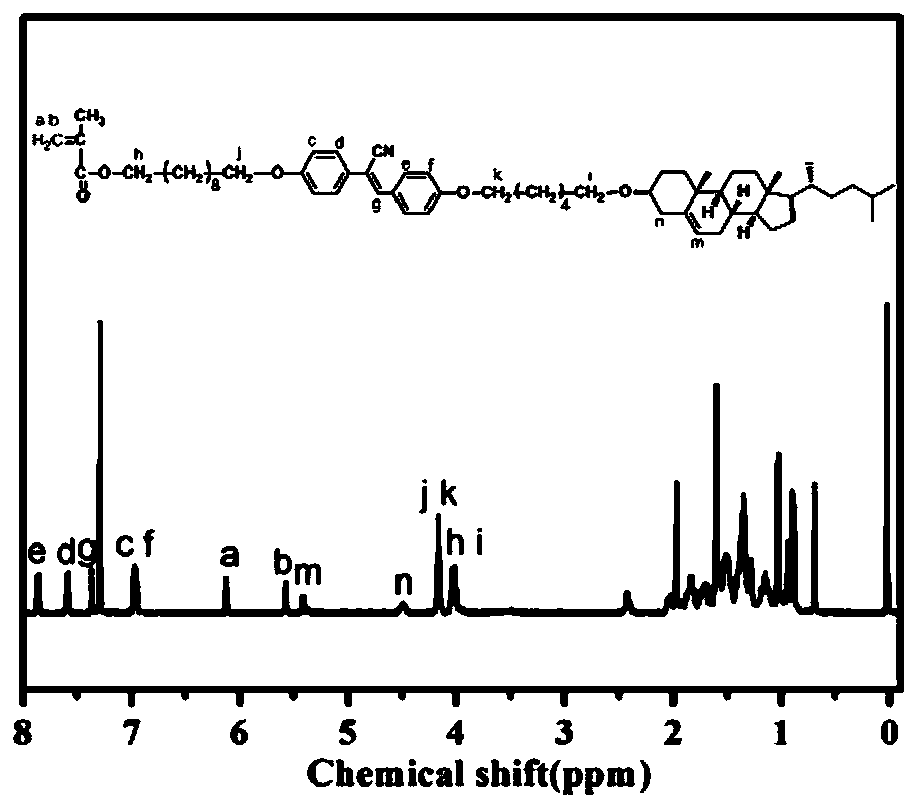
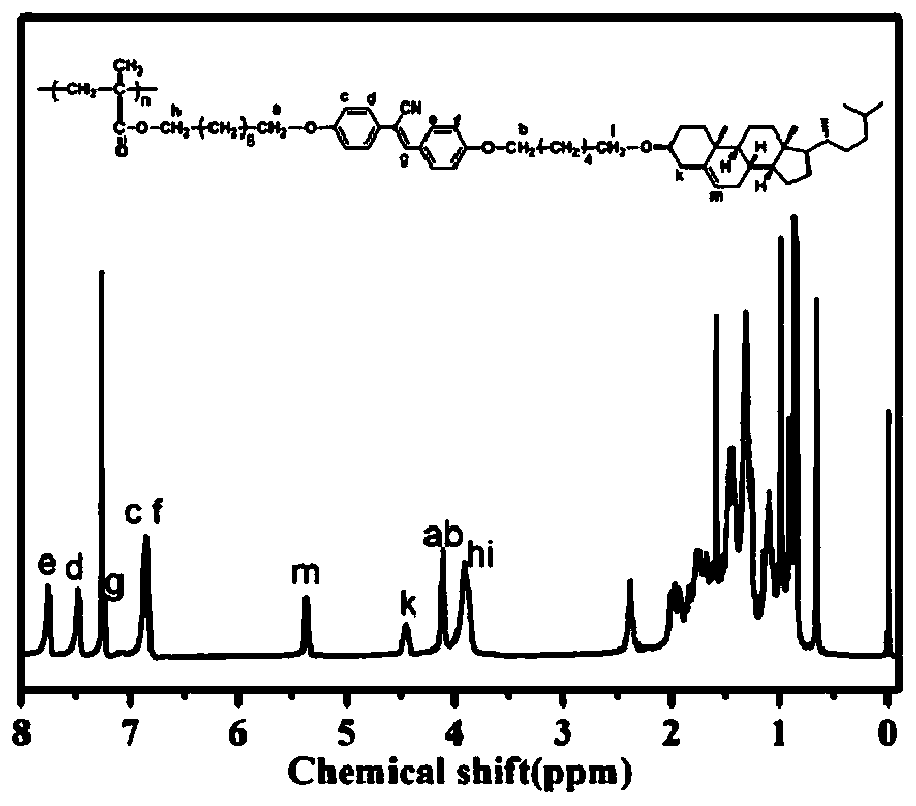
Chiral light-emitting liquid crystal polymer with circular polarization light-emitting property and preparation method thereof
A technology of liquid crystal polymers and circular polarization, applied in the direction of luminescent materials, liquid crystal materials, chemical instruments and methods, etc., to achieve the effects of rich types, obvious aggregation-induced fluorescence enhancement properties, and simple structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] (1) Synthesis of benzaldehyde derivatives
[0056] Add p-Hydroxybenzaldehyde (8.0g, 65.6mmol) and bromoethanol (10.7g, 85.7mmol) successively in the single-necked bottle, then add 100mL of DMF, stir to dissolve the raw materials, then add anhydrous potassium carbonate (27.2g, 197.0 mmol), reacted at 100 DEG C for 8h, after the completion of the reaction, suction filtered potassium carbonate while hot to obtain the crude product filtrate, and the filtrate was concentrated under reduced pressure with ethyl acetate and sherwood oil mixed solvent as eluent (wherein the volume ratio was 1.5: 1) Column separation, collecting the target component liquid, spin-drying, and obtaining a colorless liquid product after vacuum drying.
[0057] (2) Synthesis of phenylacetonitrile derivatives
[0058] P-Hydroxybenzonitrile (4.0g, 30.1mmol), 1,6-dibromohexane (14.7g, 60.2mmol), anhydrous potassium carbonate (12.5g, 90.3mmol) and 200mL of acetone were sequentially added to the single-ne...
Embodiment 2
[0067] (1) Synthesis of benzaldehyde derivatives
[0068] With the step (1) in the embodiment 1.
[0069] (2) Synthesis of phenylacetonitrile derivatives
[0070] P-Hydroxybenzonitrile (4.0g, 30.1mmol), 1,10-dibromodecane (16.2g, 60.2mmol), anhydrous potassium carbonate (12.5g, 90.3mmol) and 200mL of acetone were sequentially added to a single-necked flask, Stir to dissolve the raw materials, and reflux at 75°C for 10 hours. After the reaction was completed, the anhydrous potassium carbonate was removed by suction filtration, the filter cake was washed 3 times with acetone, the filtrate was collected, and the crude product was obtained after rotary evaporation. The crude product was washed with a mixed solvent of dichloromethane and petroleum ether (volume ratio=1:1). Remove the agent and separate through the column, collect the target component liquid, spin dry, and obtain the pure product after vacuum drying.
[0071] (3) Synthesis of cyano-stilbene derivatives
[0072] ...
Embodiment 3
[0080] (1) Synthesis of benzaldehyde derivatives
[0081] With the step (1) in the embodiment 1.
[0082] (2) Synthesis of phenylacetonitrile derivatives
[0083] Add p-hydroxybenzonitrile (4.0g, 30.1mmol), 1,12-dibromododecane (18.1g, 60.2mmol), anhydrous potassium carbonate (12.5g, 90.3mmol) and 200mL of acetone to the single-necked flask successively , stirred to dissolve the raw materials, and reflux at 75°C for 10h. After the reaction was completed, the anhydrous potassium carbonate was removed by suction filtration, the filter cake was washed 3 times with acetone, the filtrate was collected, and the crude product was obtained after rotary evaporation. The crude product was washed with a mixed solvent of dichloromethane and petroleum ether (volume ratio=1:1). Remove the agent and separate through the column, collect the target component liquid, spin dry, and obtain the pure product after vacuum drying.
[0084] (3) Synthesis of cyano-stilbene derivatives
[0085] Add ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com