Conjugated polymer and preparation method and application thereof
A conjugated polymer and polymer technology, which is used in semiconductor/solid-state device manufacturing, electrical components, circuits, etc., can solve the problem that conjugated polymers cannot meet market demands, and achieves improved device application range and high external quantum efficiency. , the effect of good luminous performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
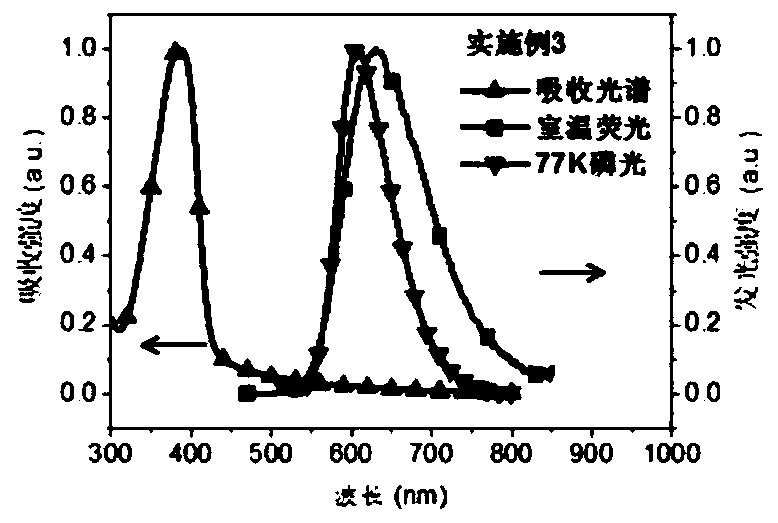
Examples
preparation example Construction
[0061] The present invention also provides a kind of preparation method of polymer described in the present invention, comprising:
[0062] Copolymerize the compound with the structure of formula (II), the compound with the structure of formula (III) and the compound with the structure of formula (IV) or formula (V) to obtain the polymer with the structure shown in formula (I);
[0063]
[0064]
[0065] Wherein, D is a C6-C50 aryl group with electron-donating ability or a C5-C50 heteroaryl group with electron-donating ability;
[0066] Ar is a C6-C50 aryl group or a C4-C50 heteroaryl group;
[0067] R 1 is H, halogen or cyano;
[0068] n is 1-200;
[0069] x is 0
[0070] According to the present invention, the present invention will have the compound of formula (II) structure, the compound of formula (III) structure and the compound of formula (IV) structure or formula (V) structure copolymerization, obtain the polymer of the structure shown in formula (I) Wh...
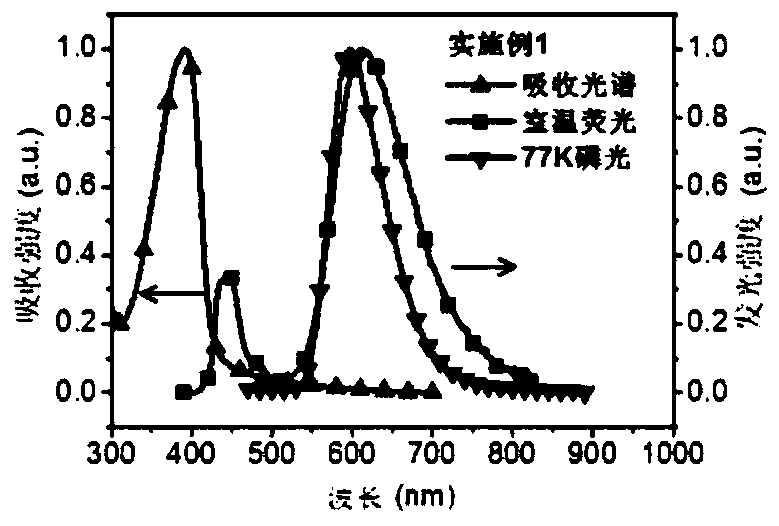
Embodiment 1
[0075] Embodiment 1: the synthesis of polymer PCzAQC0.1
[0076] (1) Synthesis of 9,9-dihexyl-2,7-dimethyl-9,10-dihydroacridine
[0077] The preparation process is shown in the following formula:
[0078]
[0079] The specific steps are:
[0080] Methyl 2-(N-p-methylphenyl)amino-5-methylbenzoate (27.9g, 109.4mmol) was added to a 500mL three-necked flask, pumped and ventilated several times, protected by argon, and anhydrous and oxygen-free The tetrahydrofuran (120mL) was stirred and dissolved, and then the newly prepared tetrahydrofuran solution of Grignard reagent hexylmagnesium bromide (156mL, 2.9mol / L) was slowly added to the reaction system. Hydrochloric acid aqueous solution (400mL, 1mol / L), extracted with ether, and the organic phase was evaporated under reduced pressure to remove the solvent to obtain the intermediate; the intermediate, 240mL glacial acetic acid and 60mL concentrated hydrochloric acid were added to a 500mL single-necked flask, refluxed and condense...
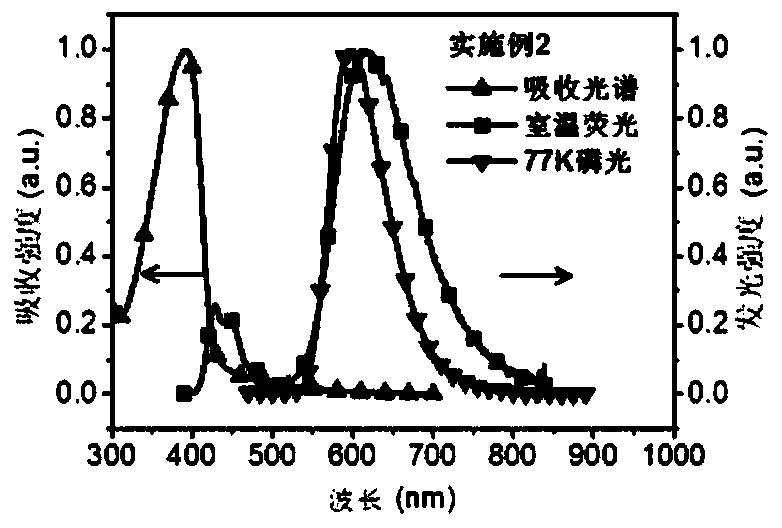
Embodiment 2
[0097] Embodiment 2: the synthesis of polymer PCzAQC0.3
[0098] The preparation process is shown in the following formula:
[0099]
[0100] The specific steps are:
[0101] Accurately weigh 5,8-dibromo-2,3-bis(4-(9,9-dihexyl-2,7-dimethylacridin-10(9 hydrogen) base)phenyl)quinoxaline- 6,7-dinitrile (1.5mg, 0.0012mmol), 2,7-dibromo-9-heptadecylcarbazole (112.0mg, 0.1988mmol), 2,7-dipinacol borate-9 -Heptadecylcarbazole (131.5mg, 0.20mmol) and bis(tris(o-methylphenyl)phosphine)palladium dichloride (1.6mg, 0.002mmol) were placed in a 50mL Schlenk bottle, and the gas was exchanged several times, Under argon protection, add anhydrous and oxygen-free tetrahydrofuran (15 mL), stir to dissolve, then add tripotassium phosphate aqueous solution (1 mL, 2 mmol / mL), heat up to 50 ° C for 0.5 h; dissolve phenylboronic acid (0.20 g, 1.6mmol) was added to the system for 5h; 0.3mL bromobenzene was added to the system for 5h; sodium diethylcarbamate (0.2g) dissolved in 10mL of water was ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com