Macrocyclic compound as well as preparation method and application thereof
A macrocyclic compound and compound technology, applied in electrodes, electrolysis processes, electrolysis components, etc., can solve the problems of catalyst electrochemical activity decline, content loss, uneven distribution of the microenvironment of the active site, etc., and achieve excellent hydrogen peroxide production performance. , the effect of improving selectivity, high concentration production potential
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0051] The first aspect of the present invention provides a method for preparing a macrocyclic compound, comprising: preparing a first macrocyclic compound by reacting a monomer with a benzyl bromide compound having a symmetrical structure;
[0052] Wherein, the monomer has the structure shown in formula I:
[0053]
[0054] The structure shown in Formula I is 2,5-bis(4-pyridine)thiazo[5,4-d]thiazole, denoted as Py 2 TTz.
[0055] In the preparation method provided by the present invention, usually, the reaction needs to be carried out in the presence of a solvent, and the solvent is selected from one or more of chloroform, dioxane, acetonitrile and N-methylpyrrolidone combination of species. In a specific embodiment, the monomer is dissolved in a solvent, and then mixed with a benzyl bromide compound having a symmetrical structure, reacted at a reaction temperature of 30-120°C, filtered after the reaction, and the precipitate is collected, and then soaked with an organic...
Embodiment 1
[0090] Step (1): Synthesis of 2,5-bis(4-pyridine)thiazo[5,4-d]thiazole monomer
[0091] In a reaction flask, 4-pyridinecarbaldehyde (0.58ml, 6.15mmol) and dithiooxamide (0.25g, 2.08mmol) were added and dissolved in N,N-dimethylformamide (10ml), nitrogen Reflux at 150°C for 6 hours under atmosphere, cool, filter, and wash the precipitate with water to obtain a light yellow powder (0.30 g, 49%), which is 2,5-bis(4-pyridine)thiazo[5,4-d]thiazole, denoted as Py 2 TTz.
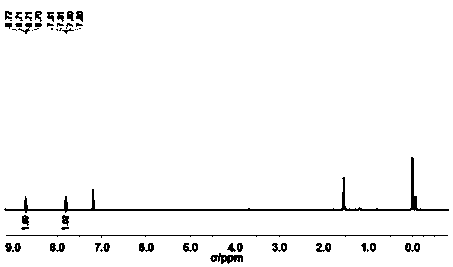
[0092] Such as figure 1 Shown, the proton nuclear magnetic resonance spectrum of product is characterized as follows: 1 H-NMR (500MHz, CDCl3 ): δ8.71(d, J=6.0Hz, 4H), 7.81(d, J=6.4Hz, 4H);
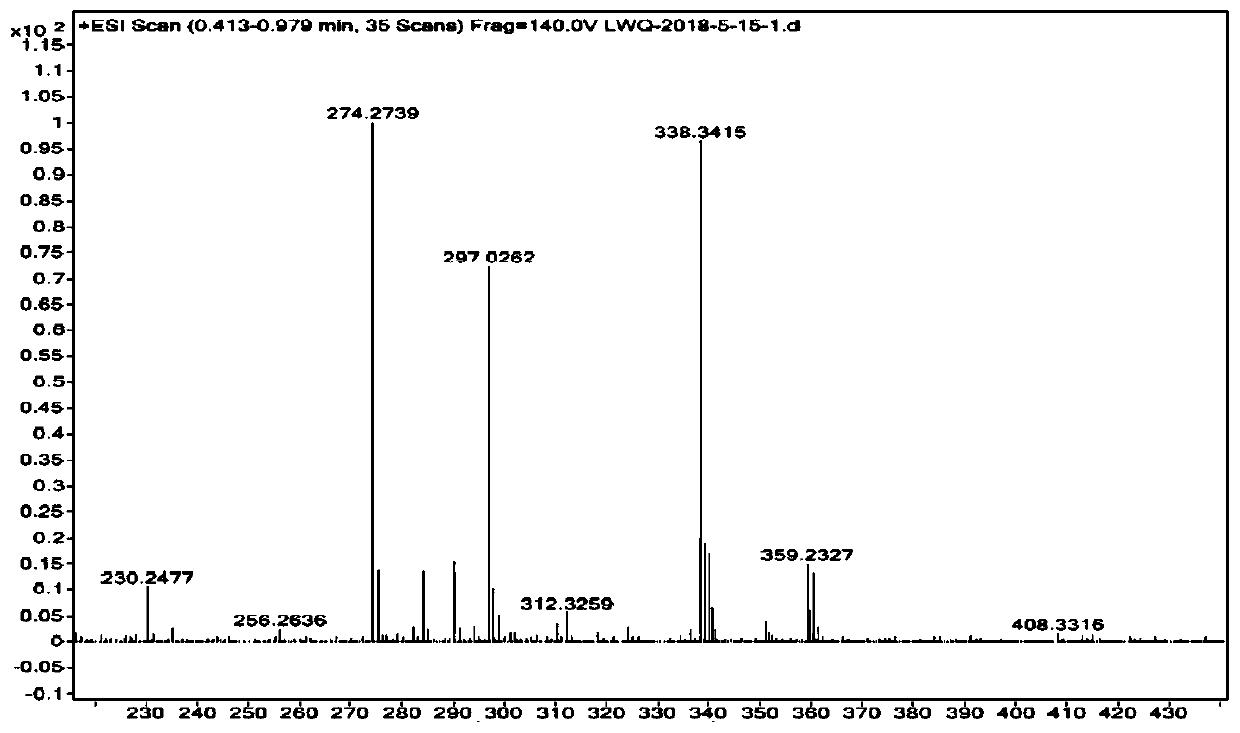
[0093] Such as figure 2 As shown, the high-resolution mass spectrometry characterization data of the product are as follows: HRMS (ESI) m / z: 297.0262.
[0094] Step (2) Preparation of the first macrocyclic compound
[0095] In a 250ml reaction vial, Py 2 TTz (0.24g, 0.81mmol) and chloroform (17mL) were added, 1,3,5-tri...
Embodiment 2
[0099] Step (1): Synthesis of 2,5-bis(4-pyridine)thiazo[5,4-d]thiazole monomer
[0100] In a reaction flask, 4-pyridinecarbaldehyde (0.58ml, 6.15mmol) and dithiooxamide (0.25g, 2.08mmol) were added and dissolved in N,N-dimethylformamide (10ml), nitrogen Reflux at 150°C for 6 hours under atmosphere, cool, filter, and wash the precipitate with water to obtain a light yellow powder (0.30 g, 49%), which is 2,5-bis(4-pyridine)thiazo[5,4-d]thiazole, denoted as Py 2 TTz.
[0101] Step (2) Preparation of the first macrocyclic compound
[0102] In a 250ml reaction vial, Py 2 TTz (0.24g, 0.81mmol) and chloroform (17mL) were added, 1,3,5-tris(bromomethyl)benzene (0.19g, 0.54mmol) was dissolved in chloroform (7ml) and added dropwise The above solution was protected by nitrogen, stirred at 30°C for 72 hours, filtered, and the precipitate was collected. Rinse with dichloromethane, repeat three times, and dry in a vacuum oven at 60° C. for 12 hours to obtain a yellow powder (0.35 g, yie...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com