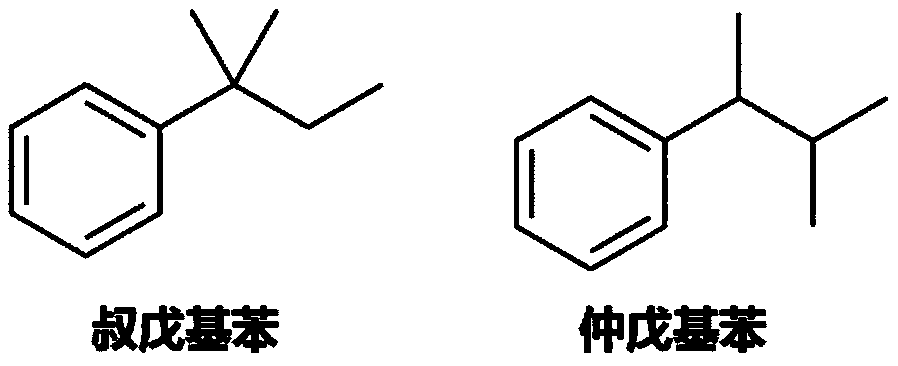
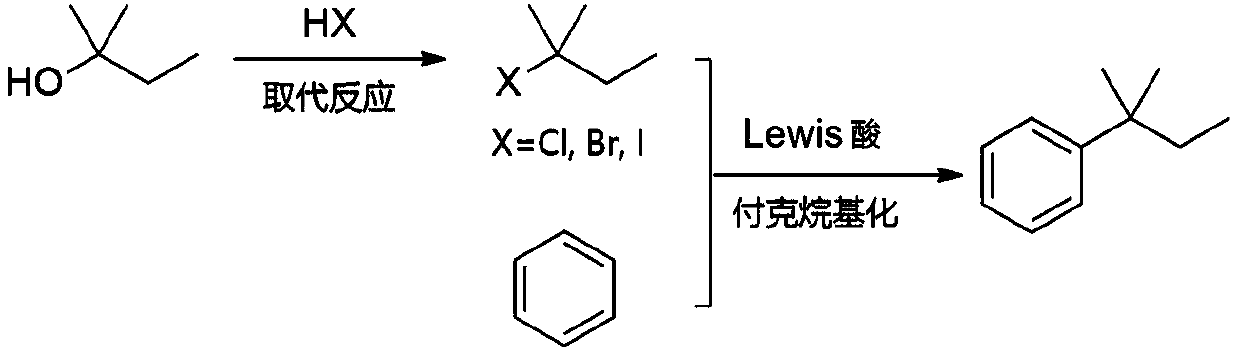
Synthesis method of tert-amylbenzene with controllable isomer content
A technology of tert-amylbenzene and synthetic methods, which is applied in the direction of chemical instruments and methods, hydrocarbons, hydrocarbons, etc., can solve the problems of affecting the yield of products, excessive local reactions, etc., and achieve fast reaction speed and catalyst The effect of small quantity and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Add 220.4g of tert-amyl alcohol into the flask, add 507.6g of concentrated hydrochloric acid (mass concentration: 36.5%) dropwise at 20°C, keep the temperature for 30 minutes after the dropwise addition, and then stop the reaction. The upper organic phase was taken, washed with deionized water, 2% sodium hydroxide solution, and deionized water respectively, and left to stand, and the organic phase was separated. After drying, 261.1 g of tert-amyl chloride product was obtained, with a yield of 98.0%.
Embodiment 2
[0028] Add 220.4g of tert-amyl alcohol into the flask, add 405.7g of hydrobromic acid (mass concentration: 47.0%) dropwise at 50° C., and then keep the temperature for 15 minutes to stop the reaction. The upper organic phase was taken, washed with deionized water, 2% sodium hydroxide solution, and deionized water respectively, and left to stand, and the organic phase was separated. After drying, 364.5 g of tert-amyl bromide was obtained, with a yield of 96.5%.
Embodiment 3
[0030] Add 80.0g of benzene and 1.31g of zinc chloride to the flask, maintain the system at 5°C, vacuumize to an absolute pressure of 50kPa, slowly add 35.1g of tert-amyl chloride and 70.2g of benzene (as a solvent, diluted Tert-amyl chloride) solution, about 1h dropwise to complete. Afterwards, the reaction was continued for 1 h at the temperature and pressure. The reaction solution was poured into 5% dilute hydrochloric acid for hydrolysis, and the organic phase obtained after liquid separation was detected by gas chromatography. It can be seen that the tert-amyl chloride was completely converted, the product yield was 97%, and the ratio of tertiary and secondary isomers was 99.32%. 0.68%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com