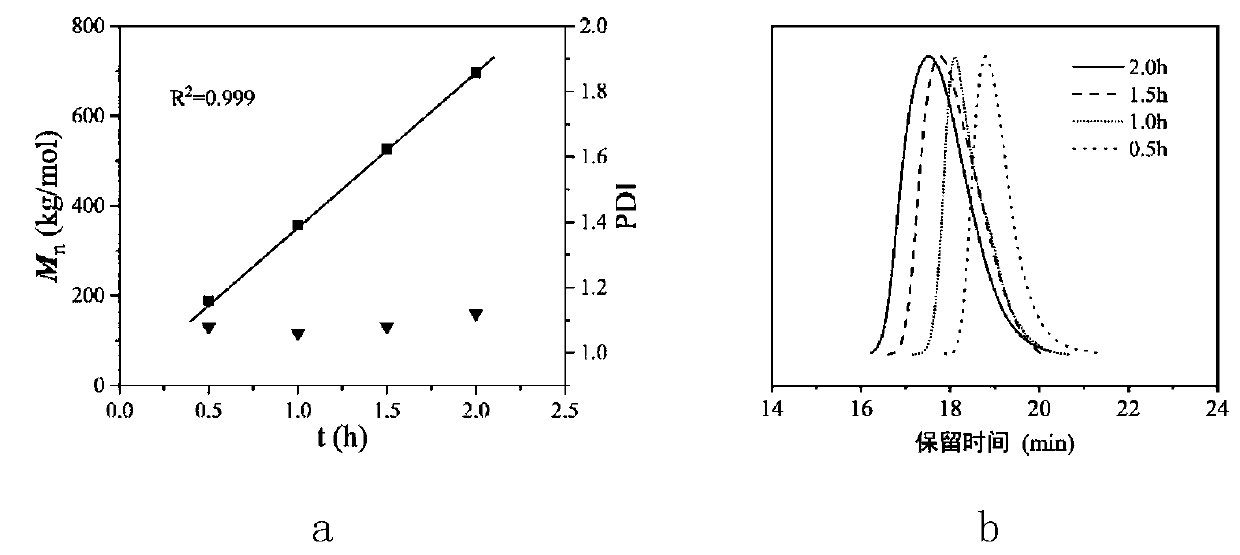
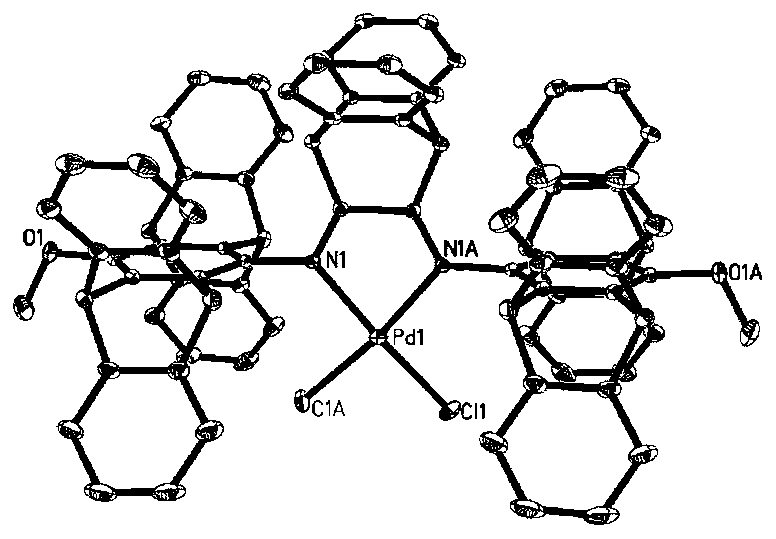
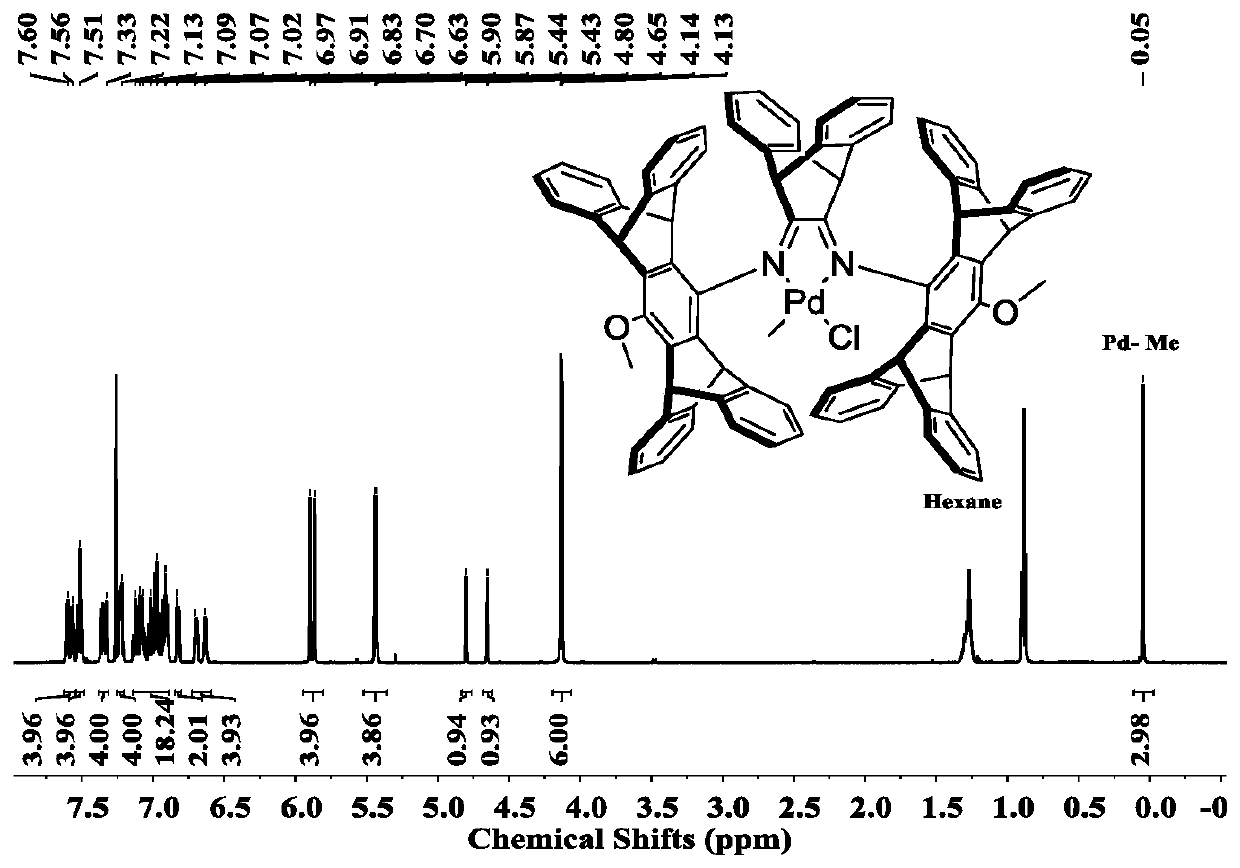
Novel benzobarrelene pentiptycene ligand, transition metal catalyst, preparation method and application in ethylene polymerization
A technology of pentaptycene and transition metal, applied in the fields of catalyst synthesis and polymer synthesis, can solve the problems of low branching degree, remain unchanged, rarely show active polymerization characteristics, catalyst temperature tolerance, etc. The effect of reduced branching degree, high activity and high temperature resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0035] The present invention also provides a preparation method of a novel benzobarrelene pentadecyl ligand, comprising:
[0036] The diketone compound with the structure (a) and the aniline compound with the structure (b) are dissolved in an organic solvent, and the organic solvent is preferably toluene, xylene, chlorobenzene, methylene chloride, chloroform or acetonitrile, and then add Stir the catalyst at 25-150°C for 6h-7 days, preferably 2-5 days, cool to room temperature, evaporate the solvent by rotary evaporation until a yellow solid appears, add excess methanol or ethanol to precipitate the product, separate the yellow solid by filtration, and use methanol or Wash with ethanol three times and dry under vacuum to obtain a class of novel benzobarrelene pentadecyl ligands described by the general formula (I). The molar ratio of the diketone compound with the structure (a) to the aniline compound with the structure (b) is 1:N, where N≥2, more preferably 1:1-100; most pref...
Embodiment 1
[0051] Embodiment 1 Synthesis of benzobarrelene pentadecyl ligand
[0052]
[0053] 1. Synthesis of 4-methoxypentyl aniline
[0054]
[0055] 4-Hydroxypentylene aniline (3g, 6.50mmol) was dissolved in 100mL of dimethylformamide, sodium hydride (468mg, 19.5mmol) was added under a nitrogen atmosphere, stirred until no bubbles were generated, then methyl iodide ( 0.6mL, 9.75mmol). Stir under nitrogen atmosphere for 1-10 days. Pour into 200mL water, extract 3-20 times with 100mL dichloromethane, separate the organic layer, dry over anhydrous magnesium sulfate for 10min-3h, filter the liquid, evaporate the solvent by rotary evaporation until a yellow solid appears, separate the yellow solid by filtration and put it in Drying under vacuum gave the product as a yellow solid (2.60 g, 84.10% yield).
[0056] 1 H NMR (500MHz, 298K, CDCl 3 ,7.26ppm): δ=7.27-7.36(m,8H,aryl-H), 6.98-6.88(m,8H,aryl-H),5.67(s,2H,CHPh 2 ),5.39(s,2H,CHPh 2 ),3.85 (s,3H,OCH 3 ) ppm.
[0057] 2. S...
Embodiment 2
[0060] Synthesis of embodiment 2 tert-butylbenzobarrelene pentadecyl ligand
[0061]
[0062] A solution of 4-methoxypentetylaniline (6.5g, 13.67mmol), tert-butylbenzobarrelene (1.9g, 5.50mmol) and p-toluenesulfonic acid (10mg) in toluene (250mL) was stirred at 145°C Reflux and maintain for 72 hours, cool to room temperature, evaporate the solvent until a yellow solid appears, add excess ethanol to precipitate the product, separate the yellow solid by filtration, wash three times with ethanol and dry under vacuum to obtain a yellow solid product (4.31g, 62.15% yield).
[0063] 1 H NMR (500MHz, 298K, DMSO-d6, 2.50ppm): δ=7.59-7.53(d, 2H, aryl-H), 7.47-7.41(d, 4H, aryl-H), 7.38-7.31(m, 10H , aryl-H), 7.16-7.11 (m, 4H, aryl-H), 7.07-6.98 (m, 4H, aryl-H), 6.91-6.80 (m, 10H, aryl-H), 6.77-6.70 (d, 2H, aryl-H), 5.88 (d, 4H, CHPh 2 ),5.34(s,2H,CHPh 2 ),5.06 (d,2H,CHPh 2 ),4.07(s,3H,OCH 3 ),1.41(d,2H,C(CH 3 ) 3 )ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com