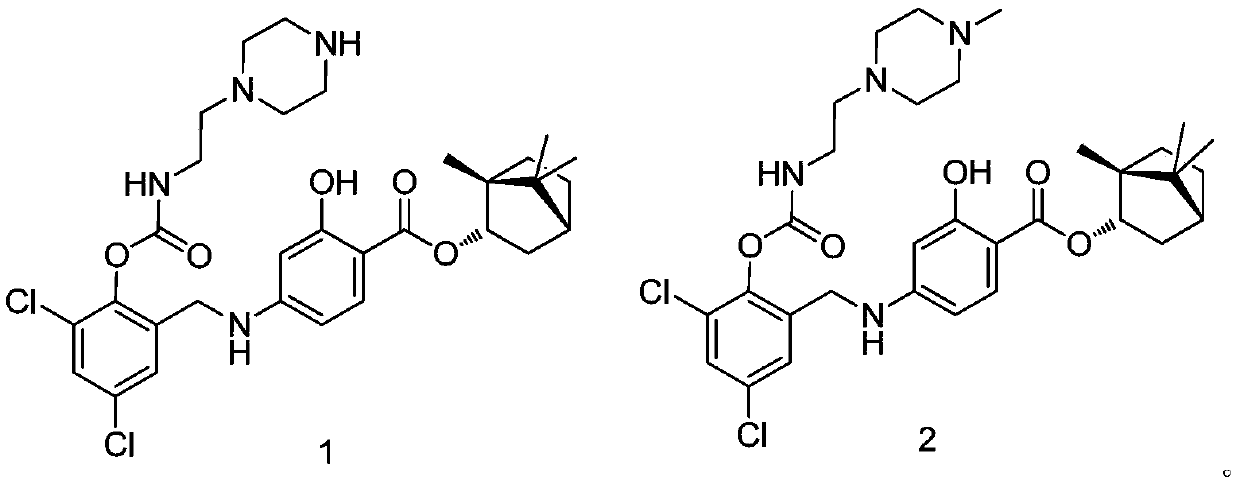
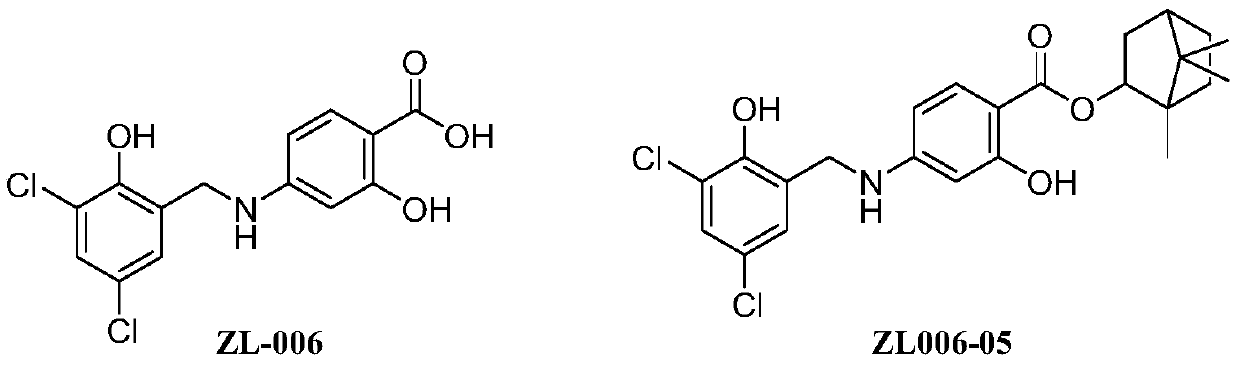
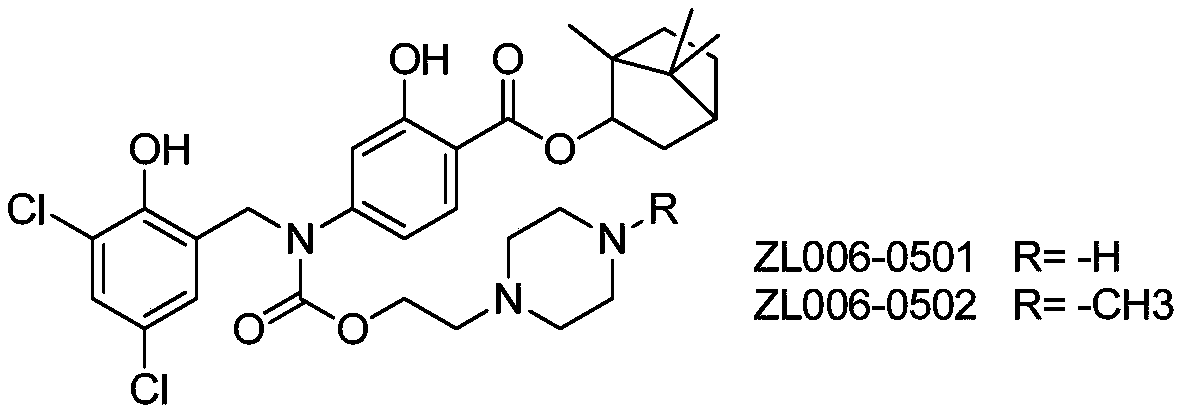
2-piperazine ethyl phenyl carbamate derivatives and pharmaceutical application thereof
A technology of phenyl carbamate and derivatives, applied in the field of 2-piperazine ethyl phenyl carbamate derivatives and their pharmaceutical uses, can solve problems such as poor water solubility, poor oral bioavailability, and unfavorable preparations , to achieve the effect of good water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Embodiment 1: the synthesis of target compound
[0018] synthetic route:
[0019]
[0020] 1.1 Synthesis of target compound 1
[0021] Synthesis of M-2: Take 3,5-dichlorosalicylaldehyde (1.9g, 10mmol), dissolve it in ethanol (10mL), add sodium borohydride (1.48g, 39.2mmol) in batches at 0°C, and react at room temperature for 2 Hours, the solvent was distilled off under reduced pressure, 30 mL of water was added, extracted three times with ethyl acetate (10 mL), the organic phase was washed with saturated aqueous sodium chloride solution and water, dried over anhydrous magnesium sulfate, and the solvent was distilled off under reduced pressure. Silica gel column chromatography (mobile phase: petroleum ether / ethyl acetate=10:1, V / V) gave product M-2 (1.7 g). The product is a white solid. 1 H NMR (400MHz, CDCl 3 )δ7.32(s,1H),7.31(s,1H),4.71(s,2H).
[0022] Synthesis of M-3: Take M-2 (1.4g, 7.2mmol), dissolve it in tetrahydrofuran (5mL), add phosphorus tribromide (2...
Embodiment 2
[0029] Embodiment 2 target compound water solubility test
[0030] At 25°C, the saturated aqueous solution (0.1mol / L sodium phosphate buffer solution) of the target compound was used to measure the main peak area by the high performance liquid phase method, and was compared with the high performance liquid phase main peak area of the methanol solution of the target compound at 0.1 mg / mL. For comparison, calculate its solubility in water.
[0031] Table 1 Solubility of target compounds in water (25°C, mg / mL)
[0032]
[0033] The water solubility test of the target compound shows that the solubility of the target compound in weakly acidic (pH=5.0-6) aqueous solution is significantly higher than that of ZL006-05.
Embodiment 3
[0034] Example 3 Determination of target compound blood drug concentration and active metabolite ZL006-05 concentration in vivo
[0035] C57 mice were administered intravenously (i.v.2mg / kg) with the same mol dose of the compound (Example Compound 1: 2mg / kg, ZL006-0501: 2mg / kg, ZL006-05: 1.5mg / kg), 5min after administration, Blood was collected at 15min, 30min, 1h, 2h, 4h, 6h, 8h, 10h, and 24h, the plasma was separated, and the concentration of the target compound and ZL006-05 in the plasma was determined by high performance liquid chromatography-mass spectrometry. The pharmacokinetic parameters of the target compound were calculated by DAS software, and the absolute bioavailability was calculated according to the ratio of the area under the drug-time curve for oral administration and intravenous administration.
[0036] Chromatographic conditions The chromatographic column is an Agilent Eclipse Plus C18 column (50mm×2.1mm, 1.8μm); the mobile phase is ultrapure water-acetonitr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com