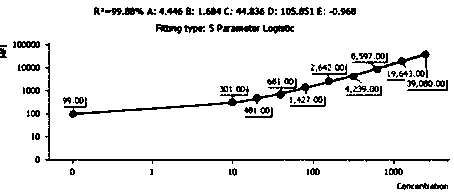
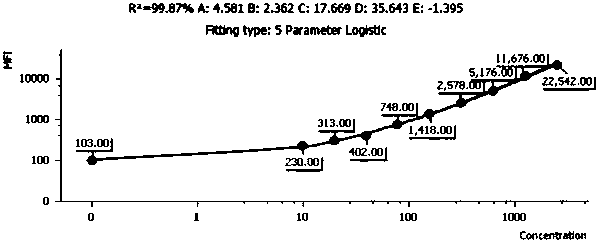
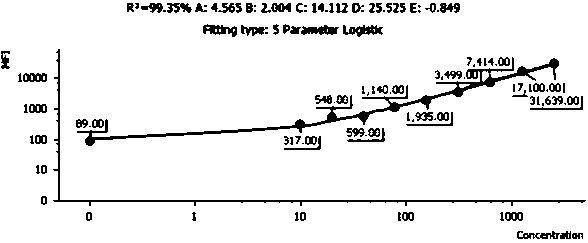
Quantitative detection method and kit for rapid diagnosis of human respiratory pathogens
A rapid diagnosis and respiratory technology, applied in the field of pathogen diagnosis, can solve the problems that the staining efficiency is greatly affected by the permeability rate, the cell culture method has a long cycle, and the transportation and storage are easy to be infected, so as to achieve high sensitivity, fast detection and time saving. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1: Rapid Diagnosis and Detection Kit for Human Respiratory Pathogens
[0051] Including human respiratory pathogen RSV antibody capture microspheres, PE-labeled detection antibody reagents, RSV recombinant protein standards, flow cytometer calibration microspheres, sample buffer, microsphere buffer, lysis buffer, washing solution. The respiratory pathogens described therein also include respiratory syncytial virus (RSV), influenza A virus (IA), influenza B virus (IB), adenovirus (Adv), parainfluenza virus type 1, parainfluenza virus type 2 and At least one of parainfluenza virus type 3, metapneumovirus, boca virus, coronavirus, rhinovirus, enterovirus, mycoplasma or chlamydia.
Embodiment 2
[0052] Example 2: Preparation of Human Respiratory Pathogen Detection Kit
[0053] The present embodiment is the preparation of a single RSV antibody detection kit for human respiratory pathogens, which is carried out according to the following steps:
[0054] 1. Preparation of RSV antibody capture microspheres
[0055] 1) Solution preparation
[0056] Preparation of buffer A: Weigh 0.96g of disodium hydrogen phosphate, add 600mL of deionized water to dissolve, adjust the pH to 6.0, add water to make up to 800mL, and filter with a 0.45um water-based filter membrane;
[0057] Preparation of buffer B: Weigh 7.5g of glycine, add 800mL of water to dissolve, adjust the pH to 7.5, add water to make up to 1000mL, and filter with 0.45um water-based filtration membrane;
[0058] 20mg / ml 1-(3-dimethylaminopropyl)-3-ethyl-carbodiimide hydrochloride (EDC) solution was prepared; 1g of EDC was weighed, dissolved in 50mL of water, and filtered with a 0.45um water-based filtration membrane;...
Embodiment 3
[0075] Example 3: Rapid Diagnosis and Detection Method of Human Respiratory Pathogens
[0076] This example is a method for detecting a single pathogen (RSV) using the kit prepared in Example 2, which is carried out according to the following steps:
[0077] 1. Drawing of the standard curve: Take the human recombinant RSV antibody standard, add 2 mL of sample buffer, let it stand at room temperature for 15 minutes, and use a pipette to gently mix the standard. At this time, the concentration of the standard is 5000 viruses per ml. Quantity; take 10 flow sampling tubes and mark the serial dilution multiples as 1 / 2, 1 / 4, 1 / 8, 1 / 16, 1 / 32, 1 / 64, 1 / 128 / , 1 / 256 , 0; add 200μL of sample buffer to each tube, take 200ul of liquid from the standard tube to 1 / 2 tube, pipette and mix well and take 200μL of liquid to 1 / 4 tube, and so on to 1 / 256 tube; Take 50 ul of the RSV antibody capture microspheres prepared in Example 2, centrifuge at 200 g for 5 min after mixing, aspirate the superna...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com