N-cyclohexyl-furan-2-formamide compound as well as preparation method and application thereof
A technology of formamides and compounds, applied in the field of N-cyclohexyl-furan-2-carboxamides, which can solve the problems that are not conducive to environmental protection, and achieve the effects of low cost, stable yield and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
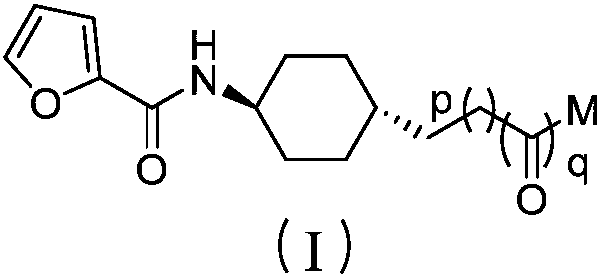
[0085] Preparation of N-(trans-4-(2-oxoethyl)cyclohexyl)furan-2-carboxamide (I-1);
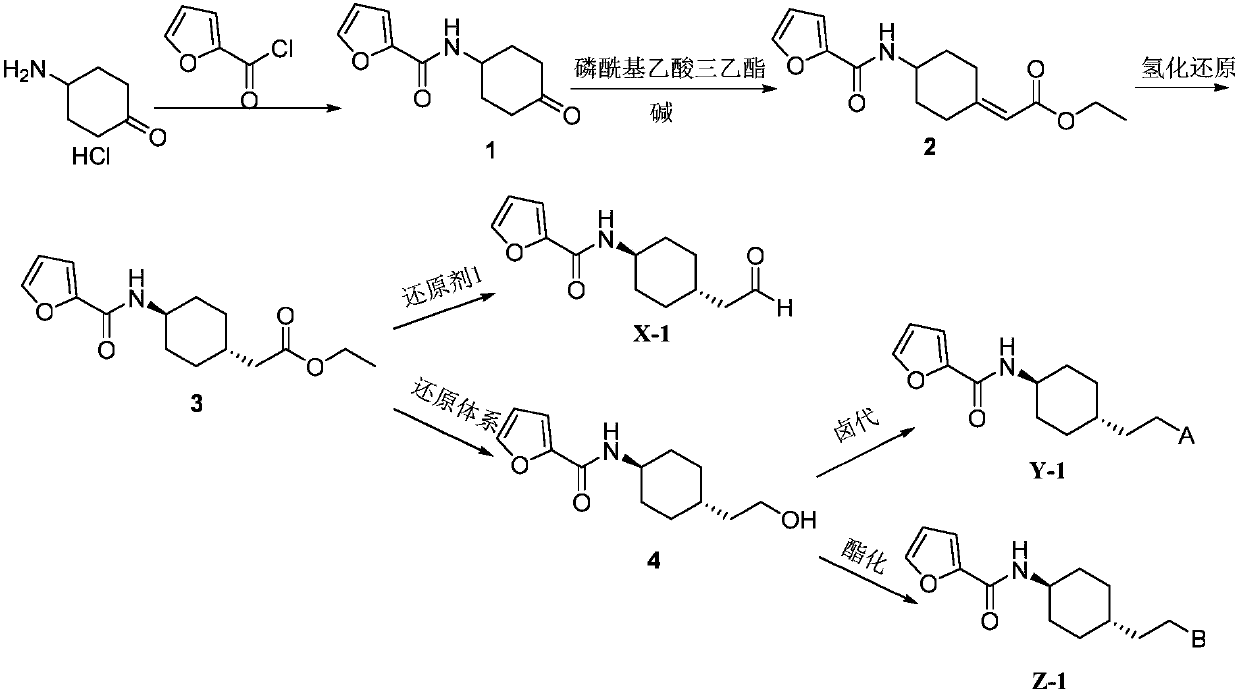
[0086] (1) Synthesis of N-(4-oxocyclohexyl)furan-2-carboxamide (1)
[0087] Add 4-aminocyclohexanone hydrochloride (1496.2g, 10.0mol), dichloromethane (7481mL), and 20% NaOH aqueous solution (NaOH: 1000g) into a 10L four-neck flask, cool to 0°C in an ice bath, and slowly Add furan-2-formyl chloride (1435.8 g, 11.0 mol) dropwise, control the temperature not to exceed 10°C, after the addition is complete, stir at room temperature for 2 h, add H 2 O (1000mL) was stirred, separated, and the organic layer was washed successively with 5% HCl (200mL×2), saturated brine (2000mL×1), anhydrous NaSO 4 Dry, filter and concentrate to obtain 1968.7 g of white solid with a yield of 95%, which is directly used in the next reaction.
[0088] (2) Synthesis of ethyl 2-(4-(furan-2-carboxamide)cyclohexylenyl)acetate (2)
[0089] Add sodium tert-butoxide (1153.2g, 12mol) and THF (3000mL) into a 10000mL four-neck...
Embodiment 2
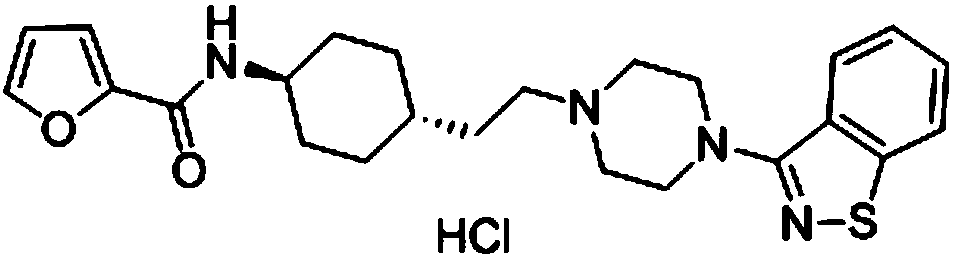
[0097] Preparation of SIPI6398
[0098] N-(trans-4-(2-oxoethyl)cyclohexyl)furan-2-carboxamide (I-1) (100.0g, 0.43mol), 3-(piperazin-1-yl)benzene Add [d]isothiazole (94.3g, 0.43mol), sodium triacetoxyborohydride (137.8g, 0.65mol), and 1,2-dichloroethane (1000mL) into a 2000mL single-necked bottle, and stir at room temperature for 15h , add potassium carbonate aqueous solution (2500mL), separate the layers, extract the aqueous layer with 1,2-dichloroethane (1500mL×1), combine the organic layers, wash with saturated brine (1000 mL×1), anhydrous sodium sulfate Dry, filter, concentrate, remove most of the solvent, filter, wash the filter cake with ethyl acetate (200mL×3), combine the filtrates, and concentrate to obtain 188.6g of white solid, yield 91%. The resulting white solid, ethanol (1800 mL), and 10% HCl (0.43 mol) were added to a 3000 mL one-necked flask, refluxed for 0.5 h, cooled to room temperature, stirred for 1 h, and filtered to obtain 171 g of SIPI6398 white solid, w...
Embodiment 3
[0103] Preparation of N-(trans-4-(2-chloroethyl)cyclohexyl)furan-2-carboxamide (II-1)
[0104] 2-(trans-4-(furan-2-carboxamido) cyclohexyl) ethyl acetate (3) (200.0g, 0.72mol), NaBH 4(83.2 g, 2.2 mol) and THF (1000 mL) were added to a 2000 mL single-necked bottle, refluxed for 0.5 h, cooled to 40 ° C, and methanol (160 mL) was added in batches, after addition, refluxed for 5 h, and the reaction solution was cooled to room temperature. Adjust the reaction solution to pH 1-2 with concentrated hydrochloric acid, stir for 0.5h, adjust the reaction solution to pH 8-9 with 10% NaOH aqueous solution, stir for 0.5h, extract with dichloromethane (500mL×3), and saturated saline (500×1) washed, anhydrous Na 2 SO 4 Dry, filter, and concentrate to obtain 158.9 g of N-(trans-4-(2-hydroxyethyl)cyclohexyl)furan-2-carboxamide as a white solid, with a yield of 93%.
[0105] Add N-(trans-4-(2-hydroxyethyl)cyclohexyl)furan-2-carboxamide (50.0g, 0.21mol) and pyridine (18.3g, 0.23mol) into a 500...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com