The preparation method of 3,4-dimethoxybenzonitrile
A technology of dimethoxybenzonitrile and dimethoxyphenylacetone, applied in the field of preparation of 3,4-dimethoxybenzonitrile, can solve the problem of low yield, good yield, high reaction temperature, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
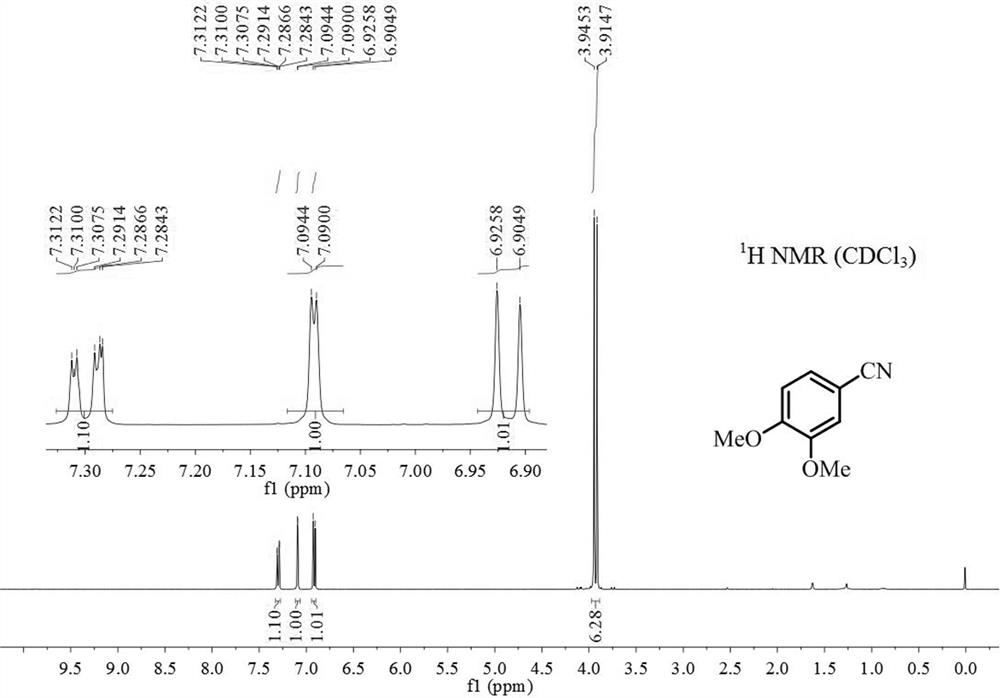
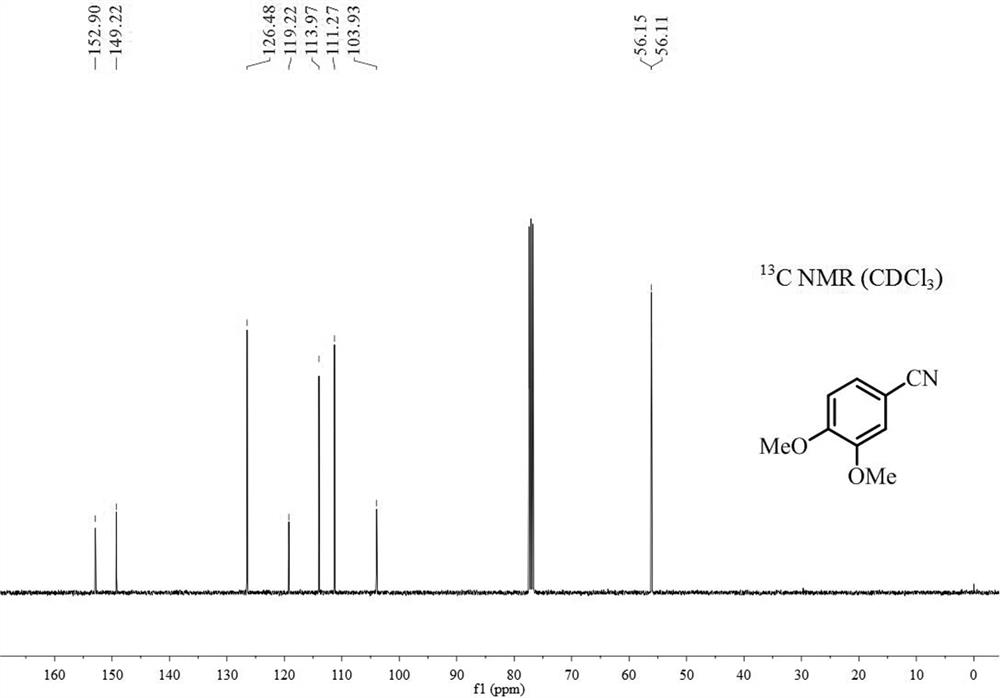
[0019] Add 0.5mmol 3,4-dimethoxyphenylacetone, 0.75mmol sodium nitrite, 0.025mmol iron complex A and 2mL dimethyl sulfoxide after dehydration and drying into the reaction tube, seal the reaction tube Reaction at 90°C for 20h (when the reaction tube was sealed, the air in the reaction tube was replaced by argon twice); after the reaction was finished, the gas chromatography internal standard method was used to carry out quantitative analysis, and the productive rate was 95%. The 3,4-dimethoxybenzonitrile product is separated and purified (mobile phase: a mixed solvent of sherwood oil and ethyl acetate with a volume ratio of 10:1), and the resulting product is detected by hydrogen spectrum and carbon spectrum, and the results are as follows: figure 1 and figure 2 shown, according to figure 1 shown 1 H-NMR and figure 2 shown 13 C-NMR confirms that the structure of product is as follows:
[0020]
Embodiment 2-3
[0022] Sodium nitrite in Example 1 is replaced with nitrogen dioxide and nitrogen monoxide in equimolar amounts respectively, and other conditions are the same as Example 1. The productive rate of 3,4-dimethoxybenzonitrile product is respectively 33% , 38%, it can be seen that when sodium nitrite is used as nitrogen oxide to react with the substrate, the yield is higher, while under the same reaction conditions, nitrogen dioxide and nitric oxide will cause the yield to decrease.
Embodiment 4-5
[0024] The complex of iron in embodiment 1 is replaced with iron trichloride, iron trifluoromethanesulfonate respectively of equimolar amount, other conditions are with embodiment 1. The production of 3,4-dimethoxybenzonitrile product The rates are 32% and 39% respectively. Due to the large difference in the spatial structure of these three catalysts, especially the spatial structure of the iron complex is quite different from that of ferric chloride and ferric trifluoromethanesulfonate, it shows a significantly higher catalytic activity than the latter two. .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com