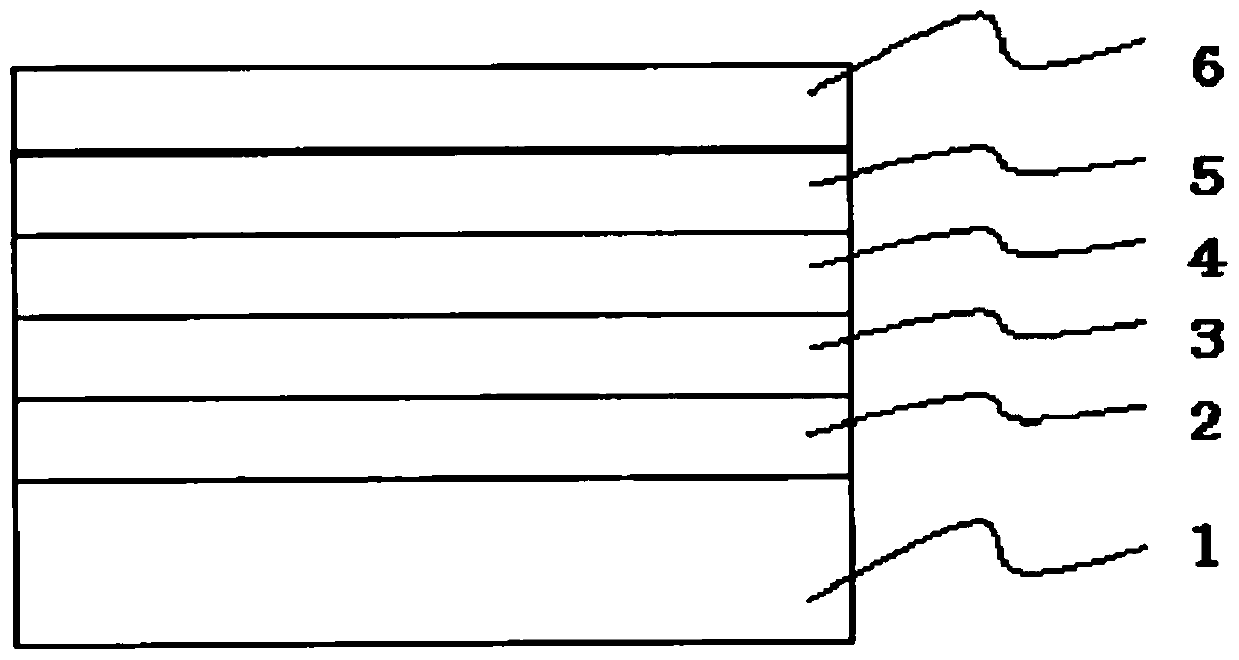
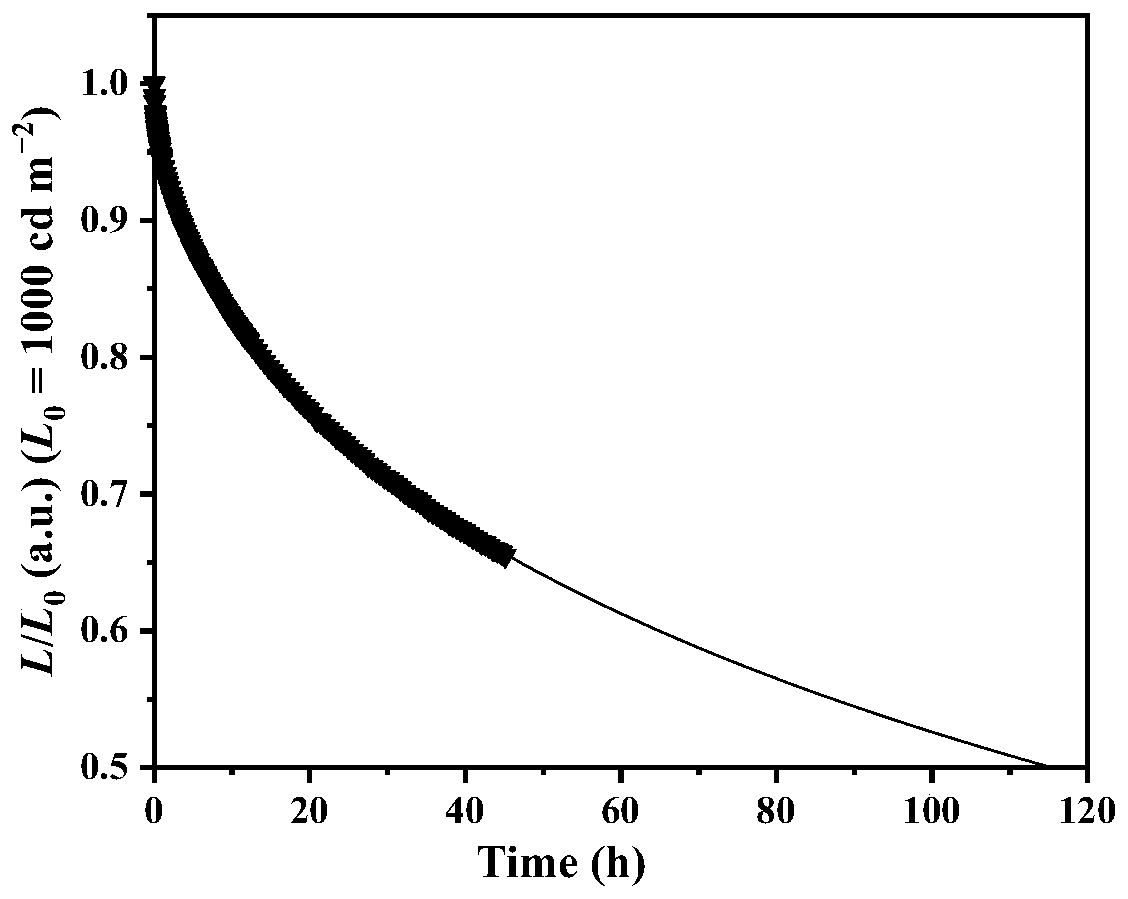
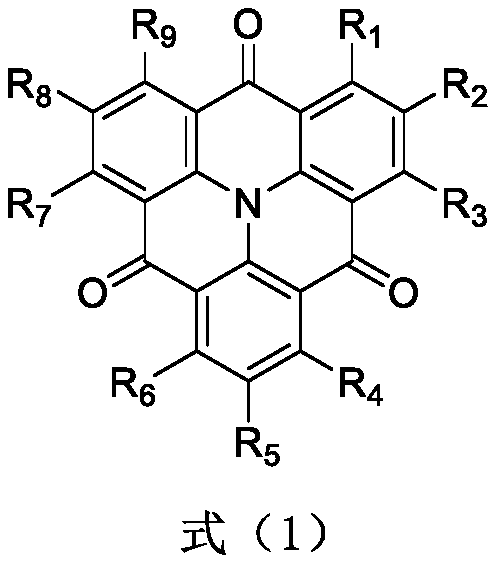
Aggregation-state luminescent material with high efficiency and narrow half-peak width
A technology of luminescent materials and aggregation state, which is applied in the direction of luminescent materials, semiconductor/solid-state device manufacturing, semiconductor devices, etc., and can solve problems such as poor color purity, difficulty in adjusting luminescent light color, and inability to meet the high color purity of full-color display technology.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1: the synthesis of compound TOAT
[0046]
[0047] Compound 1 (methyl anthranilate) and compound 2 (methyl o-iodobenzoate) can be obtained directly;
[0048] Compound 3: Under nitrogen protection, add compound 1 (3.02g, 20mmol), compound 2 (13.10g, 50mmol), activated copper powder (0.64g, 10mmol), copper iodide (0.95g , 5mmol), 18-crown-6 (0.26g, 1mmol) and potassium carbonate (16.56g, 120mmol), and then added 100mL o-dichlorobenzene solvent, heated to reflux for 48h. After the reaction was complete, cool to room temperature, filter and collect the lower organic phase, dry over anhydrous sodium sulfate, and concentrate in vacuo with a rotary evaporator. Using petroleum ether: ethyl acetate (3:1) as the eluent, it was further purified by silica gel column chromatography to obtain 4.36 g of white solid with a yield of 52%.
[0049] Product characterization: 1 H NMR (600MHz, CDCl 3 ,ppm): δ7.59(dd,J=7.7Hz,1.6Hz,3H),7.38-7.34(m,3H),7.10-7.05(m,6H),3.37(s,...
Embodiment 2
[0054] Embodiment 2: the synthesis of compound 3PhTOAT
[0055]
[0056] Compound 4: Compound 3 (3.77g, 9mmol), silver nitrate (9.17g, 54mmol) and iodine (10.28g, 40.5mmol) were sequentially added to a 500mL flask, and 300mL ethanol solvent was added, and the reaction was stirred at room temperature for 12h. After the reaction was complete, an equivalent amount of sodium sulfite solution was added until the solution was colorless, the lower organic phase was collected by filtration, dried over anhydrous sodium sulfate, and concentrated in vacuo with a rotary evaporator. Using PE:EA (5:1) as the eluent, it was further purified by silica gel column chromatography to obtain 6.17 g of white solid with a yield of 86%.
[0057] Product characterization: 1 H NMR (600MHz, CDCl 3 , ppm): δ7.91(d, J=2.2Hz, 3H), 7.64(dd, J=8.6Hz, 2.2Hz, 3H), 6.77(d, J=8.7Hz, 3H), 3.43(s, 9H ). 13 C NMR (151MHz, CDCl 3 ,ppm): δ165.58,145.75,141.17,139.54,129.27,127.64,87.05,52.12.MS(MALDI-TOF).Cal...
Embodiment 3
[0064] Embodiment 3: the synthesis of compound 3PTPTOAT
[0065]
[0066] Compound 7: Under nitrogen protection, compound 4 (1.59g, 2mmol), 4-tert-butylphenylboronic acid (1.60g, 9mmol), tetrakis (triphenylphosphine) palladium (0.31g, 0.27 mmol and potassium carbonate (2.48g, 18mmol), then add toluene (60mL), ethanol (20mL) and deionized water (9mL) as a solvent, and heat to reflux for 12h. After the reaction is cooled to room temperature, wash with dichloromethane and deionized Ion water is extracted, and the lower organic phase is collected, dried over anhydrous sodium sulfate, and concentrated in vacuo with a rotary evaporator.Using petroleum ether: ethyl acetate (5:1) as eluent, further purified by silica gel column chromatography to obtain yellow Green solid 1.49g, yield 91%.
[0067] Product characterization: 1 H NMR (600MHz, CDCl 3 ,ppm): δ7.87(d,J=2.3Hz,3H),7.63(dd,J=8.5Hz,2.4Hz,3H),7.55-7.53(m,6H),7.47-7.45(m,6H) ,7.20(d,J=8.5Hz,3H),3.41(s,9H),1.36(s,27H). 13 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| full width at half maximum | aaaaa | aaaaa |
| full width at half maximum | aaaaa | aaaaa |
| energy conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com